Overview – Male Pattern Baldness and DHT
Finasteride (brand names: Proscar, Propecia) is the only FDA approved oral medication that treats male pattern baldness (androgenetic alopecia).
Male pattern baldness is the most common cause of hair loss in men. About a quarter of the men who have this condition will begin losing their hair in their teens, before they reach 21. By the age of 80, 80% of males will experience some degree of this type of hair loss. ((Rathnayake D., Sinclair R. Male androgenetic alopecia. Expert Opin Pharmacother. 2010 Jun;11(8):1295-304))
Men who have male pattern baldness will lose their hair in a well-defined pattern, beginning above both temples and then advancing over time to form a typical “M” shape. Hair loss can also involve the crown, gradually advancing until the entire front, top, and crown are bald.
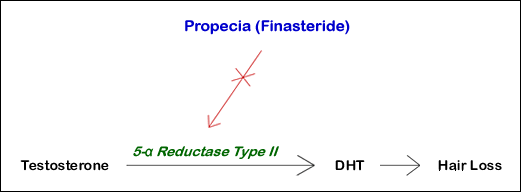
Male pattern baldness is caused by the effects of the male hormone dihydrotestosterone (DHT) on genetically susceptible hair follicles located in the front, top, and crown of the scalp.
DHT causes a susceptible hair follicle to shrink in size (miniaturize), both in diameter and length. As a result, the miniaturized follicle produces a small unpigmented vellus hair rather than the usual pigmented normal hair. DHT also shortens the susceptible follicle’s growth (anagen) phase. These two effects cause the hair to become progressively shorter and finer until it eventually disappears. (See Cause of Hair Loss in Men).
How Propecia (Finasteride) Works
Finasteride works by blocking an enzyme (5-alpha reductase) that converts testosterone to DHT.
Finasteride causes a significant drop in both scalp and blood levels of DHT and its effect is felt to be related to both of these factors. Finasteride 1-mg/day decreases serum DHT levels by almost 70%. Serum testosterone levels increased by approximately 10%, but this is within the range of normal.
History of Finasteride
It is commonly thought that finasteride was first conceived as a prostate medication and that, only by chance, was found to prevent hair loss. The fact is that in 1974 a researcher Imperato-McGinley described a group of genetically male children from the village of Salinas in the Dominican Republic who were deficient in the enzyme 5-alpha reductase. These male children had very low levels of DHT and throughout their life, their prostates remained small and they did not develop male pattern hair loss or acne.
The objective of the researchers was to find a drug that could block the 5-alpha reductase enzyme and mimic the abnormality found in these men. They could then use this drug to prevent both prostate enlargement (BPH) and hair loss. The decision was made, however, to obtain FDA approval for the medical indication first, rather than the cosmetic one. As a result, in 1992, Finasteride 5-mg was released under the brand name Proscar, for use in men over 50 with prostate enlargement (the prostate also has the type II enzyme). Five years later, in 1997, the FDA approved finasteride 1-mg/day (Propecia) for the treatment of male pattern baldness.
Efficacy of Propecia (Finasteride)
Studies have shown that after five years of treatment, 90% of men taking finasteride experienced either a growth of new hair or a halt to their hair loss. Specifically, after five years, 48% of men demonstrated an increase in hair growth, 42% were rated as having no new hair loss while the remaining 10% were rated as having lost hair. In comparison, 6% of men treated with a placebo demonstrated an increase in hair growth, 19% were rated as having no new hair loss while the remaining 75% were rated as having lost hair.
In the “Hair Count Clinical Study,” hair counts showed an average gain of 277 hairs per one-inch circle at the end of five years. These hairs were significantly larger than the fine, miniaturized hair characteristic of balding. In the “Hair Weight Clinical Study,” a 34% increase in hair weight was observed between Propecia and the placebo at 96 weeks.
Effectiveness on the Front of the Scalp
The indication for Propecia includes the treatment of hair loss in the front part of the scalp. There are published data from a controlled clinical trial of men with frontal hair loss that demonstrates improvement in this region of the scalp. Read more in our Answers blog post on the efficacy of Propecia on the front of the scalp.
Using Propecia
Propecia should be taken once daily with or without meals. Patients must take Finasteride for one year or longer before its effects in preventing hair loss and re-growing hair can be accurately assessed. Finasteride takes up to a year or more to exert its full effects in both preventing hair loss and in re-growing hair. During the first six months you may note some thinning of your existing hair. This may be due to either progression of your hair loss before finasteride has had a chance to work or some shedding of miniaturized hair that makes way for the new healthy anagen hair to grow. It is important to be patient during this period. You should continue the medication for at least one year before you and your doctor can assess its benefits.
Side Effects
Side effects from finasteride at the 1-mg dose are uncommon, but reversible. The one-year drug related side effects were 1.5% greater than in the control group. The data showed that 3.8% of men taking finasteride 1mg experienced some form of sexual dysfunction verses 2.1% in men treated with a placebo. The five-year side effects profile included: decreased libido (0.3%), erectile dysfunction (0.3%), and decreased volume of ejaculate (0.0%).
Most reported cases of sexual dysfunction occurred soon after starting the medication, but there have been reports of sexual dysfunction that have occurred at later points in time. The sexual side effects were reversed in those who discontinued therapy, and in 58% of those who continued treatment. After the medication was stopped, side effects generally disappeared within a few weeks. There have been anecdotal reports where side effects have persisted after discontinuation of therapy. This had been referred to as “Post-finasteride syndrome.”
When finasteride is discontinued, only the hair that had been gained or preserved by the medication is lost. In effect, the patient returns to the level of balding where he would have been had he never used the drug in the first place. No drug interactions of clinical importance have been identified.
See
Post-Finasteride Syndrome (PFS)
Post Finasteride Syndrome (PFS) is the term applied to reports of significant sexual, neurological and physical side effects, such as erectile dysfunction, depression, clouded thinking “brain fog,” penile numbness, penile shrinkage, and loss of libido, that persist in men who have taken and then discontinued finasteride.
A personal or family history of psychiatric illness may increase the risk of developing PFS. Some of these include; anxiety, depression, panic disorder, obsessive compulsive disorder (OCD) and Body Dysmorphic Syndrome (BDS).
Studies in progress are trying to better understand the incidence, cause and risk factors of PFS. More information on PFS can be found on the website: www.pfsfoundation.org.
See
Effects on Body Hair
When finasteride is used to re-grow scalp hair, it may also inhibit the growth of body hair. The reason is that DHT stimulates the growth of body hair in adult males and the formation of DHT is blocked by finasteride. However, the genetic variation among people is too great to know how an individual person may respond. For those who may be concerned, finasteride will not increase body hair growth. Read more about the in our Answers blog.
Adverse Reactions
Adverse reactions related to the breast, including breast tenderness or breast enlargement (gynecomastia), occurred in 0.4% of men taking finasteride 1-mg (Propecia), but this was no greater than in the control group. Other side effects that were not statistically significant included hypersensitivity reactions including rash, pruritus, urticarial (hives), swelling of the lips and face, and testicular pain.
Effects on PSA
Finasteride causes a decrease in serum PSA (prostate specific antigen) by approximately 50% in normal men. Since PSA levels are used to screen for prostate enlargement and prostate cancer, it is important that your personal physician is aware that you are taking finasteride so that he/she may take this into account when interpreting your PSA results.
Finasteride and Prostate Disease
The results of an 18-year, 18,000 patient study on prostate cancer prevention, published in August 2013 in the New England Journal of Medicine, showed that taking finasteride 5mg a day does not increase the likelihood of death from prostate cancer. Early results from the same study had suggested that finasteride might increase the risk of developing higher grade tumors; however, follow-up results from the long-term study show that men taking the drug do not have an increased risk.
Additionally, the results of the study show that taking finasteride actually decreases the likelihood of a diagnosis of prostate cancer in men by 30% and a diagnosis of “low-grade” cancer in men by 43%. By shrinking the healthy prostate tissue, finasteride may decrease the chance of a false positive result in PSA screening tests and can avoid unnecessary surgery.
Read more about the long-term study into Propecia and Prostate Disease.
Fertility
Finasteride may decrease fertility in some men. The effects may be due to changes in the composition of ejaculate and/or a reduction in sperm count. The effects appear to be reversible on discontinuing the medication.
Caution During Pregnancy
Finasteride use is contraindicated in women when they are, or may be, pregnant. Women should not handle crushed or broken Propecia tablets when they are pregnant, or may potentially be pregnant, because of the possibilities of absorption of finasteride and the subsequent potential risk to a male fetus. Propecia tablets are coated and will prevent contact with the active ingredient during normal handling, provided that the tablets have not been broken or crushed.
Exposure of pregnant women to semen from men treated with Propecia poses no known risks to the fetus.
Use in Post-Menopausal Women
Merck recently carried out a study to evaluate the efficacy of finasteride in post-menopausal women. After one year there was no significant hair growth and, as a result, the study was terminated. An explanation is that hair loss in women is related more to the action of the enzyme aromatase (which is unaffected by finasteride) rather than DHT. It is also possible that the low DHT levels observed in postmenopausal women are responsible for the lack of significant response to finasteride. (See Causes of Hair Loss in Women).
Long-Term Benefits
The effects of finasteride are confined to areas of the scalp that are thinning, but where there is still some hair present. It does not grow hair in areas that are completely bald. Although it can regrow hair in thin areas, the major benefit of finasteride seems to be in its ability to slow down or halt hair loss. Results generally peak around one year and then are stable in the second year or decrease very slightly. Although the long-term ability of finasteride to maintain one’s hair is unknown, the majority of men find that after 5 years the medication is still working.
The benefits of finasteride will stop if the medication is discontinued. Over the 2-6 months following discontinuation, the hair loss pattern will generally return to the state that it would have been if the medication had never been used.
Propecia and Hair Transplantation
Finasteride has shown to be useful in complementing a hair transplant for several reasons:
- Propecia works best in the younger patient who may not yet be a candidate for hair transplantation.
- Propecia is less effective in the front part of the scalp, the area where surgical hair restoration can offer the greatest cosmetic improvement.
- Propecia can re-grow hair, or stabilize hair loss, in the back part of the scalp where hair transplantation may not always be indicated.
For those who choose not to take Propecia, or who cannot take it due to its side effects, the surgical hair restoration is just as effective. The only difference is that medications can prevent further hair loss whereas surgery cannot. Medications are not needed for a hair transplant to be successful or the transplanted hair to grow and be permanent.
Generic Finasteride
Finasteride 5mg (Proscar) and 1mg (Propecia) are available in generic formulations. For those wanting to take the most inexpensive route, we recommend buying a pill cutter at the pharmacy and taking ¼ of a 5mg tablet every day. Remember that there is a potential risk to pregnant women from handling broken or crushed tablets.
Off-Label Dosing
There are no scientific studies that prove that increasing the dose will have any additional beneficial effects on hair loss. There are published data demonstrating that 5 mg is no better than 1 mg in controlled clinical trials. In practice, however, doctors may increase the dose when someone has been on the same dose of medication for 3-5 years and then stops responding (begins to lose hair after being stable). It has been our experience that increasing the dose may enable the medication to continue to be effective. It is important to understand that increasing the dose is an off-label use of this medication. It may increase the incidence of adverse reactions. When increasing the dose, we generally use generic finasteride 5mg that is taken whole or broken into parts (see Caution during Pregnancy).
Prostate Cancer Screening
In 2018, the US Preventive Services Task Force Recommendation Statement regarding screening for prostate cancer included the following: For men aged 55 to 69 years, the decision to undergo periodic prostate-specific antigen (PSA)-based screening for prostate cancer should be an individual one. The reason is that although it offers a small potential benefit in decreasing the morbidity of prostate cancer, there are potential risks from the testing itself. You should discuss the need for testing with your primary doctor or urologist. Routine prostate screening is not recommended for persons age 70 and older.
Common Misconceptions about Finasteride
Myth: Women can’t touch the medication.
Fact: Pregnant women should not handle crushed or broken tablets.
Myth: It only works in the crown.
Fact: It potentially works any where on the scalp where there is some hair, even in the front of the scalp.
Myth: Once you start it you must take it for ever.
Fact: You can stop the medication any time you want – you just lose its benefits when one stops.
Myth: Finasteride lowers testosterone.
Fact: The medication generally causes a rise in serum testosterone levels by approximately 10%.
Myth: Finasteride causes birth defects if a man takes it when his wife is pregnant.
Fact: Exposure of pregnant women to semen from men treated with Propecia poses no known risks to the fetus.
Myth: Propecia was originally a prostate medication that was found to prevent hair loss.
Fact: Propecia is not a prostate medication that was by chance noted to have a side effect of hair growth, it is a medication that was known since its discovery that it could grow hair.
Additional Information
To learn more about Propecia see the .
To hear answers to frequently asked questions about the use of this medication, go to the Drugs (Medications) section of the Bernstein Medical Blog.





