In 2002, the FDA approved Avodart (dutasteride 0.5mg) for the treatment of prostate enlargement in men (the medical term is symptomatic benign prostatic hyperplasia or BPH). Dutasteride is not approved for the treatment of male pattern hair loss.
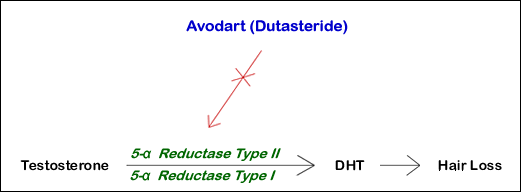
Like finasteride (the active ingredient in Propecia), dutasteride is an inhibitor of the enzyme 5 alpha-reductase responsible for the conversion of testosterone to DHT (dihydrotestosterone). However, unlike finasteride, which only inhibits the Type II form of the enzyme, dutasteride inhibits both the Type I and Type II forms of the 5 alpha-reductase enzyme. This dual effect makes the drug more potent but also increases the incidence of adverse reactions.
The Type II form of the enzyme is found predominantly in the hair follicle. The Type I form of the enzyme has been found in the scalp and sebaceous glands, but its exact role in hair growth has not been determined.
Effects on Hair Growth
Dutasteride 0.5mg/day decreases serum DHT 91% and scalp DHT 54%. Finasteride 5mg/day decreases serum DHT 71% and scalp DHT 38%. Based on these results, dutasteride should be significantly more effective in the treatment of androgenetic alopecia than finasteride. However, since the Type I form of the 5 alpha-reductase that dutasteride blocks is not present in significant quantities in the hair follicle, these effects may not be as significant as one might expect. The increased efficacy of Dutasteride is felt to be due, in part, to its effects on lowering serum DHT.
A study by Olsen et al. published in the Journal of the American Academy of Dermatology in 2006 has shown the benefits of dutasteride over finasteride in a scientifically rigorous, placebo-controlled study. See .
Side Effects
Dutasteride was investigated in controlled multi-center studies involving 4,325 men aged 50 and above with prostate enlargement. Drug-related side effects during the first six months were as follows: impotence (4.7 percent vs. 1.7 percent for placebo), decreased libido (3 percent vs. 1.4 percent), breast tenderness and breast enlargement (gynecomastia; 0.5 percent vs. 0.2 percent) and ejaculation disorders (1.4 percent vs. 0.5 percent).
The incidence of most drug-related sexual adverse events decreased with duration of treatment. The incidence of drug-related breast tenderness and breast enlargement remained constant over the treatment period. Ejaculate volume may be decreased in some patients with continued treatment. This decrease did not appear to interfere with normal sexual function.
Effects on PSA
Dutasteride will reduce the amount of PSA measured in the blood and this must be taken into account when PSA is used in the detection of prostate cancer.
Precautions
The precautions when using dutasteride for prostate enlargement are more significant than those for finasteride and include:
- Women who are pregnant or may become pregnant should not handle dutasteride because of possibility of absorption of dutasteride and subsequent potential risk to a male fetus.
- Men treated with dutasteride should not donate blood until at least six months after their final dose to prevent giving dutasteride to a pregnant woman through a blood transfusion.
- Men with an allergic reaction to dutasteride or its ingredients should not take it.
- Men with liver disease should talk to their doctor before taking dutasteride.
- Because of the potential for drug-drug interactions, care should be taken when administering dutasteride to patients taking potent, chronic CYP3A4 enzyme inhibitors (e.g., ritonavir).
Some Additional Points to Consider Regarding Dutasteride
- Unlike finasteride, where families that had a deficiency of the Type II 5-alpha reductase enzyme were followed for years without exhibiting any adverse effects from the medication, there is no natural biologic model for dutasteride.
- The half-life of dutasteride is 5 weeks compared to 6-8 hours for finasteride and serum concentrations of dutasteride are detectable up to 4-6 months after discontinuation of treatment.
Additional Information
To learn more about Avodart see the Bernstein Medical Dutasteride Information Sheet and the Prescribing Information  provided by the company.
provided by the company.
To read answers to frequently asked questions about the use of this medication, go to the Dutasteride section of Hair Restoration Answers.






