Robert M. Bernstein, MD, New York, NY and William R. Rassman, MD, Los Angeles, CA
Dermatol Surg 2006; 32: 198-204.
IT IS A SIMPLE QUESTION, one basic to hair transplantation and one constantly asked by patients. The query always elicits a quick and authoritative response by the hair transplant doctor or surgical team. To our knowledge, however, it has never been answered scientifically. The question is:
At what point are transplanted grafts so securely anchored in the scalp that they cannot be dislodged?
In other words, following a hair restoration surgery, exactly how long it will take for grafts to be so securely anchored in their recipient sites that they cannot be removed by shampooing, vigorous brushing, inadvertent rubbing or even scratching? The present study attempts to answer this basic question and to provide scientific data that can be useful in developing post-operative protocols.
The method we intended to use was rather straightforward. We planned to ask volunteers to return to the office at varying intervals after their hair transplantation procedure. At each visit we would pull on (a few of) their grafts to see if they came out. It seemed simple, but some questions immediately arose. First, how does one know by looking at a pulled graft that the growth center was extracted? In other words, was there any tissue left behind that still might be able to produce a hair? And second, what should one actually pull? Since the graft is level with the scalp surface, the only thing to grab is the hair and the scab. Should the hair transplant doctor pull the hair, the scab or both, or doesn’t it matter?
To answer these questions we first conducted a pilot study. The purpose of the pilot was to set parameters for the actual study and to determine if clinical judgment alone could be used to assess tissue viability – so that we would not have to send every specimen of every patient for histology. During the pilot, it became apparent that there were actually two types of scabs; one that stuck only to the hair and one that was adherent to the surface of the graft. (Figure 1)
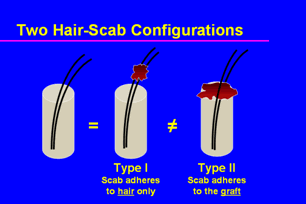
Figure 1. Hair-scab configurations
Pulling on a scab that was only attached to the hair was no different than pulling on a hair, so these situations were combined in the study. However, pulling on a scab that was adherent to the skin usually dislodged the graft – often several days after pulling on a hair was safe. (For the purposes of the study, we labeled crusts that were adherent only to hair as a Type I crusts and those that were adherent to the surface of the grafts as Type II.)
The following events were identified in the study (Figures 2-5):
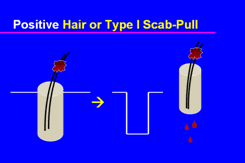
Figure 2. Positive hair-pull.
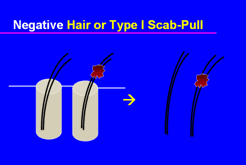
Figure 3. Negative hair-pull.
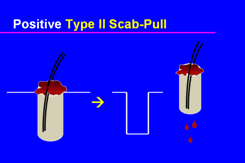
Figure 4. Positive scab-pull.
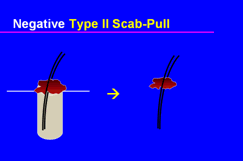
Figure 5. Negative scab-pull.
Five hair restoration patients participated in the pilot. They were asked to return every few days up to two weeks post-operatively (or until their grafts could no longer be pulled out). The average patient had four or five visits and the maximum number of visits required was ten. At each visit, we performed a series of graft pulls and sample tissue was sent for histologic evaluation. (The protocol for the graft pulls and tissue biopsies is described in the Materials and Methods section.)
The histologic evaluation of the tissue specimens from the pilot patients confirmed that what we observed clinically was accurate. In other words, it showed that by looking at the tissue we were able determine if we had removed a viable graft – or left it behind. Based on this information, we felt that we could rely on our clinical judgment in the actual study when determining if a graft had been pulled out.
In the main study, the protocol for the visit was the same but, since we had established the validity of the clinical assessment of the graft pull in the pilot, we no longer sent the extracted tissue for histology. In addition, in order that patients would not be subjected to losing multiple grafts, we asked each hair transplantation patient in the main study to return only once. In the study, 289 graft pulls were performed on a total of 42 patients.
Methods and Materials
Pilot
Five patients participated in the pilot study. Each returned to the office every 2-3 days during their post operative course for two weeks or until grafts could no longer be pulled out. In the pilot, no patient was actually required to return after 10 days, as all the grafts were secure by this time. Graft pulls were performed only on the largest follicular units, i.e. those that had been placed into 18-gauge needle recipient sites. It was felt that since these grafts would be the least secure, it would make the test the most sensitive. A fine needle holder was used to grasp the hair and/or scab. The tissue was yanked from the scalp in one quick motion. The patients experienced little or no discomfort from this manipulation and no anesthesia was required.
On each visit, three hair (or Type I scab) pulls were administered. This was followed by three Type II scab pulls. Once a graft was extracted, the test was stopped for that day. The first positive pull or the last negative pull on that day was sent for histology using Hematoxalin and Eosin staining. Biopsies were sent to Pathology Associates. P.C. in New York.
Main Study
In the main study, an additional 37 hair transplant patients were examined. These patients were asked to return at varying intervals up to two weeks post-op, but testing was performed at only one visit. Although the graft pulls were uniformly negative at 10 days in the pilot, patients in the main study were asked to return at intervals as long as two weeks post-op.
Results
Pilot
The histologic evaluation of the tissue specimens from the patients in the pilot study confirmed that the determination of whether a graft was removed or not could be determined clinically. Below are the clinical results from the first pilot patient, the histology, and pathologist’s comments. (Figures 6-9) The data from the five pilot patients can be found in the Appendix; Graft Anchoring Study: Patient Data.
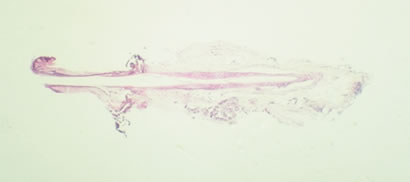 Figure 6. Day 2: Entire follicle present – Interpretation: Positive
Figure 6. Day 2: Entire follicle present – Interpretation: Positive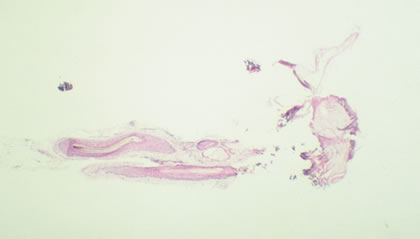 Figure 7. Day 4: Inferior portion with detached matrix and IRS – Interpretation: Positive
Figure 7. Day 4: Inferior portion with detached matrix and IRS – Interpretation: Positive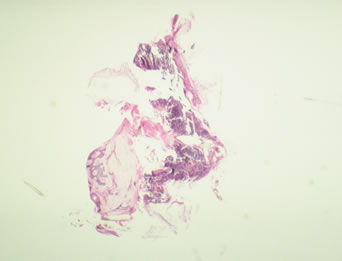 Figure 8. Day 7: Isthmus of follicle present only – Interpretation: Negative
Figure 8. Day 7: Isthmus of follicle present only – Interpretation: Negative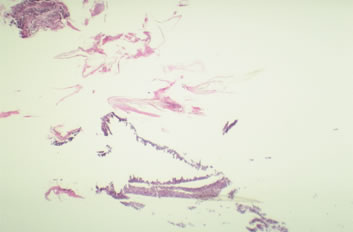 Figure 9. Day 10: Scale-crust and hair shaft – Interpretation: Negative
Figure 9. Day 10: Scale-crust and hair shaft – Interpretation: NegativeMain Study
The following graph (Figure 10) summarizes the results of the study. The complete patient data can be found in the Appendix; Graft Anchoring Study: Patient Data. The information presented in the graph and table represents the combined data from both the pilot and study patients.
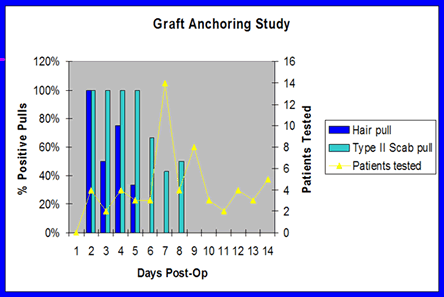 Figure 10. Summary of results from the graft anchoring study.
Figure 10. Summary of results from the graft anchoring study.The study showed that for the first two days, pulling on a hair always resulted in a lost graft, but the chance of the graft being removed started to decrease by the third day. By the sixth day pulling on a hair would no longer dislodge the graft. Pulling on an adherent scab always resulted in a lost graft through day five, with the incidence decreasing through day eight. However, by nine days post-op, grafts were no longer at risk of being dislodged.
Discussion
This study demonstrates that the presence of crusting increases the risk of grafts being dislodged in the post-op period. The implication is that if one could prevent crust formation following a hair restoration surgery, this would both shorten the time patients are at risk of losing their grafts and enable them to return to their normal hair care routines more quickly.
Although the presence of crusting increases the duration that grafts are at risk of being lost, the coagulum may serve to hold the grafts in place immediately post-op. ((Parsley WM. Management of the postoperative period. In: Unger WP, Shapiro R, eds. Hair Transplantation 4th ed., New York: Marcel Dekker, 2004:555-568.)) The challenge is to insure graft security both on the very short-term (where crusts may be of benefit) and on the longer term (where crusts have shown to be a hindrance).
A wide number of post-op protocols are currently recommended following hair transplantation procedures. They range from bandage dressings that are used to cover the entire head for several days ((Unger W. Bandaging. In: Unger WP, Shapiro R, eds. Hair Transplantation 4th ed., New York: Marcel Dekker, 2004:553-554.)) to no dressings at all.1 With the widespread adoption of Follicular Unit Transplantation there has been a trend towards using a headband to cover only the donor area the night of the procedure and then to remove all dressings the day following the surgery – leaving the transplanted area exposed. ((Bernstein RM, Rassman WR, Szaniawski W, Halperin A: Follicular Transplantation. Intl J Aesthetic Restorative Surgery 1995; 3: 119-32.)) The introduction of Follicular Unit Extraction, ((Rassman WR, Bernstein RM, McClellan R, Jones R, et al. Follicular Unit Extraction: Minimally invasive surgery for hair transplantation. Dermatol Surg 2002; 28(8): 720-7.)) where patients can resume normal physical activity more quickly after the hair transplant procedure, have increased the demand for post-op protocols that use minimal dressings.
The application of occlusive ointments can minimize crusting, but they may also make the grafts less secure immediately post-op. When an occlusive dressing is used, the covering serves to protect the grafts against inadvertent rubbing, so if there is any decreased short-term graft security from the lack of crusting, it may not be a practical issue. This may be particularly true for grafts transplanted to the top of the scalp (rather than in the temples or the donor area) where grafts are less likely to be dislodged.
The use of copper-peptide moist dressings (GraftCyte) has been used to hydrate the area, as well as provide micronutrients to speed the healing of the follicles. The value of copper-peptide in Follicular Unit Transplantation procedures, where the recipient wounds are needle-sized, has been questioned by a number of experienced practitioners.2 In addition, the inconvenience and expense of the dressings has limited its widespread use.
Since crust formation only occurs in the 1-2 day post-op period, while there is still active exudate, vigorous irrigation during this period should be able to eliminate surface crusting while allowing the coagulum that formed around the graft, to hold it firmly in place. To this end, these authors are currently instructing patients to take very frequent showers, as often as every three hours the day following hair restoration surgery. As anticipated, this one day treatment seems to significantly decrease the amount of crusting that is formed. Once patients pass through this “exudative” period, crusts no longer develop and they are free to shampoo and shower at their regular schedule. Since there is no crusting, patients are then at least theoretically able to resume their normal hair care activities such as shampooing, brushing and styling by day 6 or, if one wants to be more cautious, at one week.
As suggested above, an alternative post-op protocol would be to use occlusive dressings for the first one to two days post-op to prevent the formation of crusts. If an occlusive dressing with ointment is utilized within the first 24 to 48 hours, the duration of the exudative phase can be reduced and, there may be very little need for vigorous irrigation later.
The present study is limited in that only one size recipient site was examined, i.e. that made with an 18-g needle. It is anticipated that smaller sites will hold grafts more securely.3 With any size site it is important that the grafts have a snug fit, as this will help to hold grafts in place, facilitate healing and decrease the amount of exudate. Grafts that are trimmed very closely will be more poorly anchored compared to a plump graft placed in the same size site. It is also important to look at patients who may have significantly altered connective tissue, such as those with long standing solar elastotic change, as this would inhibit this snug fit and make graft anchoring more tenuous. Patients with significant sun damage were not addressed in this study, and this is an important group that needs to be examined.
Different types of post-operative protocols still need to be evaluated with respect to their ability to minimize crusting, particularly in the immediate post-op period. In particular, graft security during the first two days post-operative needs to be examined in controlled studies to determine the optimal regime. It may be that different parts of the scalp require slightly different protocols during this period.
Regardless of the particular method of post-operative wound care that is ultimately adopted, this study offers a methodology for the evaluation of one of the most important aspects of post-op care – that of preserving the integrity of follicular units that have been placed into the recipient sites by the hair transplant doctor. Hopefully, the limited information provided by this paper will be a first step in providing a scientific foundation for how we advise our patients in caring for their transplanted grafts.
Acknowledgements: Histologic evaluation of the tissue specimens was performed by Damien DiCastanzo, M.D. of the Pathology Associates, P.C. We greatly appreciate his assistance in this study. We also thank Nancy Hansen, Barbara Werner and Shannon Gadino for their help in data collection and the preparation of this manuscript.