Robert M. Bernstein, MD, William R. Rassman, MD, David Seager, MD Walter P. Unger, MD, FRCP(C), Bobby L. Limmer, MD, Francisco Jimenez, MD Jose M. Ruifernandez, Joseph F. Greco, PhD, PA/C, James Arnold, MD, E. Antonio Mangubat, MD, Albert J. Nemeth, MD, Jung-Chul Kim, MD, PhD Jennifer Martinick, MD, Edoardo Raposio, MD, FICS, Leonard M. Patt, PhD Marty E. Sawaya, MD, PhD, Angela M. Christiano, PhD, Emanuel Marritt, MD
Robert M. Bernstein, M.D. Assistant Clinical Professor of Dermatology, College of Physicians and Surgeons, Columbia University, New York, NY
Journal of Aesthetic Dermatology & Cosmetic Dermatologic Surgery 1999; 1(1): 55-89.
Abstract: The 1990’s have witnessed major changes in hair transplantation, most notably the trend towards the use of large numbers of very small grafts and the emergence of follicular unit transplantation as possibly the new “gold standard.” This article reviews the new developments that will shape hair restoration surgery as we enter the next millennium. Many aspects of hair transplantation will be explored including: the follicular unit/mini-micrograft controversy, ways to measure and maximize the donor supply, new concepts in graft storage, advances in wound healing, new instrumentation, automated graft cutting and placing, advances in laser technology, the role of new medical treatments, and finally the status of research in cloning and genetic engineering.
Introduction
Robert M. Bernstein, MD
The 1990’s have witnessed major changes in hair transplantation, most notably the trend towards the use of large numbers of very small grafts and the emergence of follicular unit transplantation as possibly the new “gold standard.” In addition to improved surgical techniques, new developments in medical treatments, marketing on the part of physicians, and coverage by the media, have produced an increased public awareness of this rapidly evolving field.
The long-term observations of patients treated with the older hair transplant techniques and the use of new objective means to measure donor supply, have made the modern hair restoration surgeon more keenly aware of the importance of maximizing the patient’s finite donor reserves. As a consequence, new ways of harvesting and dissecting donor tissue have been developed to better preserve this supply, and new ways of handling and storing grafts awaiting placement have been devised to enhance the viability of the grafts. In addition, long-term planning has assumed greater prominence in surgical decisions. It is somewhat ironic that after four decades of wrestling with supply/demand issues, new medications for hair loss may soon necessitate a complete re-thinking of the way we plan our procedures.
The use of large number of very small grafts has made the transplant process much more laborious and this has prompted the development of new technologies to automate various aspects of the transplant process. Long transplant sessions requiring greater numbers of staff and involving the movement of large numbers of small, fragile grafts has also made quality control a central issue.
The decade has seen a dramatic decline in the popularity of scalp reductions and flaps, and fortunately the “pluggy look” that was once the hallmark of many older transplant procedures is now deemed to be unacceptable. Unfortunately, many patients still carry the telltale cosmetic deformities caused by the older techniques and “repair work” has become an increasingly larger part of many physicians’ practices.
The initial excitement over laser assisted hair transplants appears to have subsided, because the very tiny sites needed in the newer procedures seems to be best made by cold steel incisions. However, laser technology is rapidly changing and this tool may still have a future role in hair transplantation, possibly in ways we have not yet considered.
The development of 5-alpha reductase inhibitors and other medications that specifically attack the biochemical pathways involved in androgenetic alopecia will have a profound influence on the future of hair restoration surgery. Once drugs are able to successfully limit the extent of balding, supply/demand ratios will change, long-term planning may become less important and the aesthetic demands of all patients may substantially increase. As medications become more effective, their long-term safety profile established, and their use more widespread, it is possible that baldness may be preventable. When this happens, surgery may be reserved for those already bald or for persons without significant androgenetic hair loss who want to improve upon their natural attributes.
Although hair loss in women is generally a far more significant cosmetic problem than in men, a much smaller proportion of women are surgical candidates, since they generally exhibit a diffuse type of hair loss. When medications are developed that are useful in women, an entire new population of patients may benefit from surgery. The increase in female patients, might then more than offset any decrease in the number of procedures performed in men.
Cloning is another technology that has made significant progress in recent years and may supply the surgeon with an unlimited source of donor hair. Genetic engineering, on the other hand, is a technology still in its infancy, but that may someday render the hair transplant surgeon’s role obsolete.
The move towards smaller grafts in the 90’s has produced a number of controversies that are receiving a great deal of attention in the hair transplant community. Among the most hotly debated are 1) the “supremacy” of the follicular unit over traditional mini-micrografting, 2) the practicality of microscopic dissection, 3) the importance of single strip harvesting, and 4) the “ideal” transplant density. As this decade draws to a close, however, no issue is possibly more critical to the future direction of surgical hair restoration as the debate over economy vs. quality.
The newer hair transplant techniques have enabled the surgeon to produce unprecedented naturalness, the ability to complete the process in a smaller number of sessions, and the means to accomplish this with a more limited amount of donor tissue. However, these newer procedures are technically more difficult, require a significant number of well-trained staff, are more costly to deliver, and are too impractical for some cosmetic surgeons to perform. These limitations have stimulated a number of enterprising physicians to try to facilitate the more tedious aspects of the procedure with the use of automated devices.
Although much of the new technology has served to speed up the procedure, some mechanized devices accomplish this at the expense of quality and the preservation of donor tissue. To what degree this occurs, and what its clinical significance may be, still needs to be assessed in well-controlled, scientific studies. Until then, the subjective value that surgeons and their patients place upon each of these aspects of the transplant may ultimately define the type of procedures offered over the next few years.
As we approach the beginning of a new millennium, it is tempting to speculate about the future of hair restoration surgery. This article has been a collaborative effort to review many of the new developments that will most likely shape this future. The various opinions expressed in this article do not necessarily reflect the views of all the contributing authors and some areas covered are so new that their practical value is not yet known. The purpose of this article is intended to be provocative rather than dogmatic. We trust that the reader will enjoy our exploration into the future keeping this in mind.
Follicular Unit Transplantation – The End of the Evolution?
Robert M. Bernstein, MD
After four decades of evolution from the large plugs of the late 1950’s to the extensive mini/micrografting of the early 1990’s, possibly the central development in hair transplantation today is the recognition that the naturally occurring, individual follicular unit may represent the ideal way in which to transplant hair. The underlying tenet of follicular unit transplantation is that the follicular unit is sacred and should always be transplanted intact.1 While not all hair transplant surgeons agree on the importance of using follicular units exclusively for the entire transplant, or in every patient, the central role of this previously unrecognized anatomic structure in modern hair restoration surgery is without dispute.
Follicular units are distinct groupings of usually one to four, and occasionally five terminal hairs, surrounded by a circumferential band of collagen called the “perifolliculum.”2 It also includes one, or rarely two, vellus follicles, the associated sebaceous glands, the insertions of the arrector pili muscles, and a neuro-vascular plexus. It has been demonstrated that hairs seperated from other hairs of a follicular unit do not grow as well as the same number of hairs transplanted keeping follicular units intact.3 The follicular unit is thus a physiologic, as well as an anatomic entity.
Follicular unit transplantation offers the surgeon the unique ability to transplant the maximum amount of hair with the minimum amount of non-hair bearing skin. In this way, recipient wounds may be kept small, healing is facilitated, and with proper technique, large numbers of grafts may be safely moved per session. The use of these discrete anatomic units also helps to ensure that the cosmetic result of the transplant will appear completely natural.
In contrast to follicular units, micrografts (1-2 hairs), and mini-grafts (3-6 hairs), are small grafts cut randomly from donor hair, not specifically as individual intact follicular units. They may consist of partial follicular units, single follicular units, multiple follicular units, or multiple, partial follicular units.4 In mini-micrografting, the partial units may be at risk for sub-optimal growth, and the multiple units will contain extra skin that will demand larger recipient sites. This, in turn, causes more wounding to the recipient bed and may limit the number of grafts that can safely be transplanted in a session.
It has often been said that with multiple sessions, minigrafts can look fairly natural in patients with ideal hair characteristics. However, even in these circumstances, on close inspection, minigrafts can look unnatural compared to follicular units. As the public becomes increasingly more discriminating, the future of hair transplantation is therefore likely to involve an increasing demand for procedures that are performed using follicular unit transplantation exclusively.
It is felt by most surgeons who perform follicular unit transplantation routinely, that single strip harvesting and complete stereo-microscopic dissection are required to properly dissect follicular units from the surrounding tissue4. The reasons for this are relatively straightforward. Harvesting with a multi-bladed knife will break up follicular units and transect follicles, whereas removing the donor tissue as a single strip will yield the highest proportion of intact follicular units. Once the single strip has been removed, the stereomicroscope, with its 10x magnification and intense illumination, will provide the best visualization for the dissectors to accurately subdivide the strip and to isolate the individual units. Lower power loop magnification does not provide sufficient resolution for precise follicular unit dissection and back-lighting will not penetrate the intact strip.5
Although, it is hard to argue the supremacy of the follicular unit in theory, in practice, follicular unit transplantation is tedious, demanding on the physician and staff, and requires a relatively high degree of expertise to be properly performed. It is, therefore, reasonable to assume that in situations where follicular unit transplantation is impractical or impossible, the patient might be better served by a more simple technique. In this vein, the standard practice of mini-micro grafting is seen by some as a more practical alternative to follicular unit transplantation.
The advantages of mini-micrografting are that it is faster and requires less staff. In addition, it is felt that the damage produced by the multi-bladed knife (used in mini-micrografting) is somewhat offset by the advantage of not having to carefully trim around follicular units, which in itself can be a source of follicular injury (if not done with care). On the other hand, in mini-micrografting, the slightly larger grafts and concomitantly larger wounds do not permit the total naturalness that is achieved with follicular unit transplantation. In addition, the split follicular units and greater number of hair fragments (produced by the use of the multi-bladed knife and less precise dissecting techniques) may result in less than optimal growth.
The important factors affecting graft survival are still controversial. Graft trauma can take multiple forms. Do longer transplantation procedures lead to greater graft desiccation, donor tissue anoxia (time-out-of-body) and lower yield or does violating the follicular unit microanatomy lead to a lower yield? These important questions lack the controlled studies required for meaningful answers, but the future direction of hair transplantation surgery may, in part, depend upon their outcome!
As we will discuss in subsequent sections, new technology may soon substantially change how both follicular unit transplantation and mini-micrografting are performed. However, regardless of how the technical parameters of each procedure evolve, the debate of follicular unit transplantation vs. mini-micrografting will undoubtedly hold the attention of the hair transplant community for years to come. In the meantime, the answer to which procedure is used may unfortunately lie less with the needs of the individual patient, than with the resources and capabilities of the operating surgeon and his staff.
References
0. Bernstein RM, Rassman WR, Szaniawski W, Halperin A: Follicular Transplantation. International Journal of Aesthetic and Restorative Surgery 1995; 3: 119-132.
1. Headington JT: Transverse microscopic anatomy of the human scalp. Arch Dermatol 1984;120: 449-456.
2. Seager D. Binocular stereoscopic dissecting microscopes: should we use them? Hair Transplant Forum Int 1996; 6(4): 2-5.
3. Bernstein RM, Rassman WR, Seager D, Shapiro R, et al. Standardizing the classification and description of follicular unit transplantation and mini-micrografting techniques. Dermatol Surg 1998; 24: 957-963.
4. Bernstein RM, Rassman WR. Dissecting microscope versus magnifying loupes with transillumination in the preparation of follicular unit grafts. A bilateral controlled study. Dermatol Surg 1998; 24: 875-880.
Larger Grafts – “Back to the Future?”
Walter P. Unger, MD, FRCP(C)
Is there a role for grafts larger than individual follicular units or small follicular families in hair transplantation? In at least one of the “authors” view the answer is yes, and the reason is that it is felt that more density can be obtained by using larger grafts. Limmer has reported using follicular grafting alone and achieving densities of 81 hairs per sq. cm1,2. I can achieve over 200 hairs per sq. cm with standard grafts3. Since cosmetically excellent results are possible with 60-80 hairs per sq. cm, and one can never be 100% sure of the ultimate donor/recipient ratio, more density is not a reasonable goal for a majority of patients but this does not mean “all” of them.
The use of grafts that contain more than 2 or 3 follicular units (or families), is generally limited to 10 to 15% of the patient population who a) want greater density, b) can afford greater density in terms of long term donor/recipient area ratio (usually because of an appropriate combination of family history, patient age and a positive physical examination), c) ideally have some persisting hair in the recipient area so any potential for transitory plugginess is minimized, and d) ideally have advantageous hair characteristics such as fine texture and light color, to minimize any possibility of transitional plugginess. Given the above parameters, very little transitional plugginess, if any, is noted by patients or people who see them and therefore the “price” of transitional plugginess is eliminated from the “price” the patients have to pay for this greater density. A zone of micrografting and minigrafting is always used anterior to the larger grafts to create a more natural looking hairline zone and to help camouflage the larger grafts. In addition larger grafts are never used in the posterior half of a patient with Type V or greater male pattern baldness (MPB).
Individuals who choose to use larger grafts must also be aware that, theoretically at least, the area treated with larger grafts must eventually be filled solidly (when all original hair has been lost). If not, when the hair is parted through the transplanted area, when it is wet or wind blown, plugginess may be noted. However, the density is usually so great that this is generally unnecessary even for relatively demanding patients.
The other type of patient in whom standard grafts are advantageous, are individuals who have been treated with standard grafts in the past and have obvious spaces between them with a so-called “Barbie-doll” appearance. If there is good hair density in the standard grafts on either side of a hairless space, the most efficient way of eliminating that space is to fill it with an appropriate sized graft containing hair of similar density to the flanking standard grafts. Punching out a portion of some of the larger grafts can be most useful as an adjunctive technique for this problem, rather than an alternative one.
Although it has been claimed that good hair yield cannot be accomplished if standard grafts are used5, Nordstrom showed a 90% survival6 and Unger showed a 90-110% survival7 in studies carried out many years ago. In addition, although it has been said that solid filling with standard size grafts is impossible, this has not been the experience of everyone. The argument that you need great expertise and practice to accomplish similar results and therefore most people cannot create this, is a self-fulfilling prophecy. One should aim for this level of expertise rather than abandon any attempt of achieving it.
Even physicians, who are generally follicular unit transplant enthusiasts, are again trying the use of larger grafts, containing more than one follicular unit, to produce more density. With time they may develop the confidence to try still larger grafts in properly selected patients. It is the hope of at least one of these authors that this trend will continue, so that the value of larger grafts once more becomes more generally recognized.
References
0. Limmer B: The Density Issue in Hair Transplantation. Dermatol Surg 1997; 23: 747-750.
1. Limmer B: The Density Issue, A debate that refuses to die. Hair Transplant Forum
Intl. 1998; 8(3): 1,4-6.
2. Unger W: Different Grafts for Different Purposes. Amer Jour Cos Surg 1997; 14(2):177-183.
3. Unger W: Graft types. Hair Transplant Forum Intl. 1998; 8(3): 14-16.
4. Seager D: Comments. Hair Transplant Forum Intl. 1998; 8(3): 16-17.
5. Nordstrom REA: Evaluating the Efficacy of the punch hair grafting technique. J Dermatol Surg Oncol 1989; 15: 404-408.
6. Unger W: Hair Counts: A Scientific approach to the evaluation of various factors in the survival of hair transplants. Hair Transplantation, 1st Edition, Unger W(Ed), Marcel Dekker Inc., 1979, Appendix E, P. 203-210.
Maximizing the Donor Supply
Bobby L. Limmer, MD
Since all hair restoration procedures involve the redistribution of preexistent hair, the donor supply remains an important limiting factor in hair restoration surgery. For this reason, it is absolutely essential to preserve the maximum number of viable hair follicles in every procedure. Any avoidable cause of follicular wastage should be unacceptable to both the physician and the patient. Combining the follicular sparing aspects of single-bladed donor harvest, microscopically magnified dissection of follicular unit grafts, and careful, gentle implantation methods, consistently produces a growth rate of 90-95% of the harvested hair1. Although only a relatively small percentage of hair transplant surgeons currently utilize optimal harvesting techniques, this number should increase as the importance of preserving the donor supply is more widely recognized.
The Donor Area
The punch autograph technique of Orentreich2, the multi-bladed knife excision method of Vallis3, and the single bladed donor harvest of Uebel4 have individual advantages and disadvantages. Simple mathematical analysis of the length of the surgical excision required to harvest 10 cm square area of donor tissue clearly reveals that the punch technique requires 5 times as much surgical margin as a single ellipse, and the multi-bladed knife (utilizing four blades with 3 mm spacing) requires 2.5 times as much excisional margin as a single bladed knife5. When the mathematical advantages of such significantly less surgical margins are combined with the advantage of direct intra-operative visualization of the single bladed knife excision throughout the excision process, the use of single-bladed knife for the donor harvest obviously becomes the most follicular sparing method.
It has been the experience of some of these authors that when comparing the percentage of hair follicles that are transected by multi-bladed vs. single bladed knife harvest reveals a significant difference in follicular transection rate. The single bladed knife will transect approximately 2-3% of all follicles in total donor tissue harvested compared with 12-16% follicular transection rates for multi-bladed knives utilizing 4 blades spaced at 3 mm widths between blades. When the blade spacing is decreased, the amount of transection increases dramatically.
Graft Dissection
Historically, grafts for transplantation have typically been “cut to fit” specific recipient site and sizes. Such “cut to fit” methods ignore the natural growth pattern of follicular units as they occur within the donor’s scalp. Microscopic dissection and implantation of naturally occurring follicular units as the basic graft methodology used since 1988 by Limmer6 has the advantage of not only preserving the maximum number of undamaged hair follicles for transplantation but also has the advantage of generating small, clean grafts which allow for much denser packing. In the hands of an experienced assistant, the transection rate during graft production is characteristically less than 3% of all follicles in the donor tissue.
Cooley and others7 found the transection rate to be approximately twice as high when utilizing back lighting and loupe magnification techniques compared with the dissecting microscope. Additionally, Bernstein and Rassman8 have shown that even partial microscopic dissection of the donor tissue produces a 17% greater yield of hair for transplantation when compared with loupe dissection with transillumination. If total microscopic dissection were compared, certainly the percentages of hair available for transplantation would be significantly increased above this 17% figure, probably on the order of an additional 5-10%. Most importantly, the high resolution and intense illumination of the microscope allows for the dissection of an intact donor strip and obviates the need to “pre-cut” the strip with a multi-bladed knife in order to make the subsequent dissection easier and thus eliminate this source of follicular injury as well.
References
1. Limmer BL: Survival of Micrografts. In: Hair Replacement. Stough DB, Haber R. St Louis Mosby Press 1996; 147-149.
2. Orentreich N: Autografts in Alopecias and Other Selected Dermatological Conditions. Ann. N.Y. Acad. Sci. 1959; 83: 463.
3. Vallis CP: Surgical Treatment of the Receding Hairline. Plast Reconstr Surg 1968; 33: 247.
4. Uebel CO: Micrografts and Minigrafts: A New Approach for Baldness Surgery. Ann Plast Surg 1991; 27: 476-487.
5. Limmer BL: The Donor. In: Hair Replacement. Stough DB, Haber R. St Louis: Mosby Press 1996; 142-143.
6. Limmer BL: Elliptical Donor Stereoscopically Assisted Micrografting as an Approach to Further Refinement in Hair Transplantation. Dermatol Surg Oncol 1994; 20: 789-793.
7. Cooley JE: Follicle Trauma in Hair Transplantation: Prevalence and Prevention. Presented at Int Soc Of Hair Restor Surg, 5th Annual Meeting, Barcelona, Spain. Oct 15-19, 1997.
8. Bernstein RM, Rassman WR: Dissecting Microscope vs Magnifying Loupes with Transillumination in the Preparation of Follicular Unit Grafts. A Bilateral Controlled Study. Dermatol Surg Dermatologic Surgery 1998; 24: 875-880.
New Ways to Quantify Hair Movement
Francisco Jimenez, MD and Jose M. Ruifernandez
Since hair transplantation is essentially a redistribution of a limited amount of hairs from one circumscribed area of the scalp (donor area) to another (recipient zone), the whole process of hair movement can be mathematically modeled. The advantages of this mathematical analysis are:
1. To develop an objective method of surgical planning that is reproducible.
2. To evaluate the efficiency of a hair transplant by comparing the final result with the proposed theoretical estimations.
3. To save as much donor hair as possible due to its finite supply.
Distribution of Hair in the Donor Area
Human hair emerges from the scalp in groupings containing one to four and rarely five hairs. In the average Caucasian individual, most of the hair follicles emerges from the scalp as two hair follicular units (approximately 55% are two hair follicular units, 35% are three, four and five hair follicular units, and 10% are one hair follicular units). However, there is a clear correlation between the hair density (number of hairs per square centimeter) and the relative proportion of one to four hair follicular units. For instance, individuals with average and high hair density have more numbers of three hair follicular units than one hair follicular units, but individuals with low hair density (less than 130 hairs/cm2) have more one hair units than three hair units.
These correlations can be expressed with the following mathematical equations, which are explained in greater detail elsewhere1:
n = 0.29 x d + 27
n1= -0.16 x d + 36
n2= 0.19 x d + 9
n3= 0.26 x d – 18
Where,
d = hair density (number of hairs /cm2)
n = follicular unit density (total number of follicular units / cm2)
n1= number of one hair follicular units / cm2
n2= number of two hair follicular units / cm2
n3 = number of three, four, and five hair follicular units /cm2
Note: Due to the scarcity of four and five hair follicular units and for the sake of simplification, all four and five hair follicular units are considered as three hair units.
After measuring the hair density with a densitometer, or an equivalent quantifying instrument, the hair transplant surgeon can use these equations to calculate the follicular unit density and the expected proportions of the hair groupings. The results obtained by the former equations are “most probable median values” for a specific hair density. As occurs with any statistical mathematical model applied to a biological system, a small percentage of error can be expected. Significant variations in the hair distribution (hair density and follicular density) have been noted among different races2, which make the above equations useful only for Caucasians.
The distribution of the hair in one- to four-hair follicular units is what mainly determines the great genetic variability in hair density, rather than the actual spacing of follicular units, which is relatively constant. There is also a dynamic change in the proportion of follicular units due to the progressive hair loss pattern associated with androgenetic alopecia.
The early stages of androgenetic alopecia are marked by the progressive diminution of hair shaft diameter and a decrease in length, caused by a shortening of the hair growth cycle. These changes seem precede actual hair loss. It appears that as male pattern baldness progresses, the hair is lost as single hairs from all types of follicular units, in the same proportion. Therefore, the three hair units will be converted into two hair units, the two hair units into one-hair units, and some of the one-hair units gradually disappear, although new one-hair units will be formed from preexisting two hair units. As a result (although the hair density varies significantly), the total number of follicular units per square centimeter remains between certain limits until the individual reaches a significant degree of alopecia3.
Evaluation of the Recipient Site
To measure the recipient area is very important for planning a hair transplant session. Depending on the area in square centimeters to be transplanted, the surgeon can predict the number of recipient sites to be made and, subsequently, the number of follicular units needed from the donor area.
A simple method to measure the recipient area is to use a flexible and transparent calibrated reticula that accommodates to the curved shape of the scalp. A more accurate instrument, however, could be an electronic planimeter, which by outlining the borders of the recipient zone can give us the exact measurement of the area. As far as we know, this instrument has not yet been adapted for use in hair transplantation.
The density of a hair transplant (number of hairs transplanted per square centimeter) is always an object of debate in meetings and scientific journals. We believe it is a mistake to link density exclusively with graft size (the argument is made in favor of larger grafts producing more density than smaller grafts), and that a much more critical factor is the number of recipient sites made per unit area (or how close the follicular units are placed)4. A recent in vivo study by Limmer5 compared the density achieved using the mini-micrografting technique with that accomplished by standard grafts, showing that comparable or even better density could be obtained with the mini-micrografting approach (average density of 64 hairs/cm2 after four plug sessions versus an average density of 61 hairs/cm2 along the first centimeter of frontal hairline after only 1 session of mini-micrografting).
To achieve densities in the order of 60 hairs/cm2 with randomly placed follicular units, it is necessary to create from 25 to 40 recipients sites per square centimeter depending upon the donor density of the patient and the size of follicular units used in a specific area. In adition, when using follicular units, the surgeon can dictate the density not only by changing the number of recipient sites made per square centimeter, but also by planning the distribution of the follicular units. For example, larger follicular units might be placed in those areas where maximum density is desired, such as in the central forelock. If the larger 3- and 4-hair follicular units are sorted and used in select areas, it is relatively easy for an experienced surgical team to achieve these densities with only 20 sites/cm2.2
When discussing hair transplant density, an effort should be made to always present numerical data (number of recipient sites made per square centimeter and the average number of hairs placed in each site), while avoiding nonspecific terms such us “dense packing,” which only adds confusion.
“Apparent Density” and Computer Imaging
Besides the “real density” (number of transplanted hairs per square centimeter as discussed above), there is another factor that plays an important role in the final cosmetic result and in the illusion of density, which is what we call the “apparent density.” The apparent density depends upon a number of factors including the actual number of hairs per cm2, hair shaft diameter, wave, color, skin/hair color contrast, the emergent angle of the hair, and a number of less tangible factors such as intrinsic oiliness or integrity of the hair shaft cuticle. Some of these factors may play an even greater roll in the apparent density than the absolute number of hairs per se.6
An instrument that is able to measure the “apparent” density still needs to be developed. It is obvious that a computer system that just takes into account the total number of hairs transplanted could not possibly predict aesthetic results of the surgery. In theory, if the total number of hairs transplanted, the resultant density per unit area, hair shaft diameter, hair color, wave, skin color, and styling, could all be accounted for, then computer imaging might accurately represent what a transplant might achieve in a particular individual.
Computers are certainly going to play a more significant role in the future of hair transplantation by assisting the surgeon in the overall surgical planning and in the quantification of the hair moved in a transplant. They can be used as measuring tools as well as imaging systems. Using a video digital computer system, the patient’s recepient zone can be captured on the screen. Then, the computer would measure the area, and the surgeon could draw different areas in the recipient zone, assigning a real density to each one of them. In addition, the donor site could be scanned by the computer, obtaining the hair density, the follicular density, and other salient features that would affect the outcome of the surgery. After integrating all these data, the computer could give the surgeon a number of recommendations such as:
0. The size of donor strip to be excised.
1. The number of incisions to be made in the recepient zone or in the different areas in which the receptor zone was subdivided, according to the real hair density proposed.
2. The estimation of the final apparent density based on the real densities assigned to the recepient zone or to different areas of the recepient zone.
3. A virtual image representation of the final outcome according to the proposed alternatives. Hopefully, the time for computers to reach this level of sophistication is close at hand.
References:
0. Jimenez F, Ruifernandez JM: Superficial distribution of human hair in follicular units: a mathematical model for estimating the donor size in follicular transplantation. (Submitted for publication to Dermatol Surg)
1. Bernstein R, Rassman WR: The aesthetics of follicular transplantation. Dermatol Surg 1997; 23: 785-99.
2. Bernstein RM, Rassman WR, Szaniawski W, Halperin A: Follicular transplantation. International Journal of Aesthetic and Restorative Surgery 1995; 3: 119-132.
3. Stough D, Jimenez F, Potter T: A redefinition of male pattern baldness. Dermato Surg 1996; 22: 484-5.
4. Limmer BL: The density issue in hair transplantation. Dermatol Surg 1997; 23: 747-50.
5. Bernstein RM: Measurements in Hair Restoration. Hair Transplant Forum Intl. 1998; 8(1): 27.
Controlling The Human Factor in Graft Injury
Joseph F. Greco, PhD, PA/C
In 1984, Drs. Shiell and Norwood first described the existence of X-factor1 as something producing unexpected and unexplained poor results in 4-mm grafts that occurred in less than 1% of the cases. It was conjectured that some sort of antibody reaction rejection process produced localized graft ischemia.2
The emergence of the megasession in the early 1990’s brought new reports and concerns of no growth and poor growth in large micrografting sessions. In 1994, Greco postulated the existence of H-factor as the primary cause of decreased micrograft survival. H-factor would be any “subtle or invisible iatrogenic trauma to the follicular growth center” that occurred during the operative process, resulting in decreased growth or no growth.3 The H-factor can occur during or between any phase of the transplant procedure. The effects may be categorized as either primary or secondary H-factor.
In primary H-factor, the insult is directly caused by the surgeon or the assistant. Dr. Seager nicely demonstrate this with his “skinny” and “chubby” micrograft study,4, which demonstrated decreased growth in the over aggressively dissected follicular units. Dr. Seager conjectures that poor growth also results from the “disruption of the physiological and anatomical bond between the hairs of naturally existing follicular clumps.”5
Another example of primary H-factor demonstrates that “crush injury”6 to follicular growth centers during the placement of grafts caused a decreased rate of growth in roughly handled micrografts. Drs. Cooley and Vogel clearly demonstrated the effects of delayed or no growth when the dermal papilla was traumatized at a weak point just above the papilla during harvesting, dissecting and placement.7
Secondary H-factor occurs as a result of predisposing follicular units to damage. Dr. Gandelman demonstrated a decreased growth in micrografts when follicular units were exposed to the damaging effects of drying and increased temperature.8 Additionally, if a surgeon does not provide adequate hemostasis when densely placing follicular units, bleeding may cause secondary H-factor, because the units may be over-handled by assistants during the placement phase.
Additional studies on micrograft survival by Dr. Limmer,9 tissue hypoxia studies by Dr. Goldman,10 and reduced oxygenation studies by Drs. Kemp and Kansted,11 address various causes for decreased micrograft yield in megasessions. Because of these and other studies that pinpoint the etiology of H-factor, technical advances have led to a “quantum leap” in micrograft survival in total micrografting sessions. Advancements have come in each phase of the hair transplant procedure, as well as tissue care throughout the transplant procedure.
The trend away from small strips toward the elliptical excision has dramatically decreased overt follicular transection. Interestingly, this is a return to the original megasession procedure, or “punctiform method”, developed by Dr. Carlos Uebel in 1986.12 There have been many attempts to automate the micrograft dissection process with varying degrees of success. However, most automated sectioning developments subjected harvested tissue to H-factor.
The development of back-light technology with loop magnification for transilluminated microdissection, as first reported by Dr. Paul Rose,13 allowed better visibility of light colored follicular units when compared to traditional methods of sectioning. However, the greatest advance in reducing H-factor during micro-dissection was the introduction of the stereo microscope by Dr. Limmer. 14 Recent studies by Dr. Bernstein and Rassman,15 indicate an increase in follicular unit grafts, as well as 17% increase in the total amount of hair harvested from the donor strip when using the dissection microscope as compared to magnifying loops with transillumination. Their studies show this while examining only the later phases of dissection. The differences are more significant when the microscope is used for the entire dissection process.
The introduction of chilled Petri dishes to keep the dissected grafts below room temperature has led to increased graft survival. Handling grafts in the proper anatomical zones, avoiding the lower to mid-portion of the follicle containing the dermal papilla and epithelial cells,16 avoiding over-handling and rough handling between each phase, and meticulous attention to detail by the entire team, has led to decreased incidents of H-factor.
The movement towards 16- and 18-gauge needles, as well as small lancets from the punches, has had a direct relationship in decreasing recipient site trauma and increased follicular yield in the densely placed follicular unit transplantation sessions. There are currently first generation automated placement instruments,17 so time and critical review will determine the effectiveness of automating the placement phase and reducing H-factor.
In sum, H-factor in total micrografting sessions has decreased with:
0) the development of finer placement instrumentation
1) improved placement techniques
2) overall attention to the negative effects of rough handling
3) attention to the negative effects of exposure to air and temperature
4) the use of microscopes for dissection and magnification for placement
5) an overall awareness and attention to detail during each phase of the transplant procedure.
In the final analysis, the advancements in effectively reducing H-factor since the early 1990’s have been effective because of the numerous, previously mentioned studies and the collective thinking and action of numerous hair surgeons. Advanced technology, such as computer generated scanners for dissecting follicular units, may make it possible to automate the dissecting process, while preserving the integrity of the follicular unit, thus reducing H-factor even further. Second and third generation automated graft placement instruments will speed up the graft placement phase, as well as reduce follicular trauma. Regardless of technology, the most important and effective measure in controlling H-factor now and in the future is attention to detail throughout every phase of the hair transplant procedure.
References
0. Norwood, O.T., Schiell, R.C.: Hair Transplant Surgery, 2nd edition. Springfield, IL: Charles C. Thomas, 1984.
1. Schiell, R.C.: Whither the X-factor. Hair Transplant Forum Intl. 1996; 6(4): 13.
2. Greco, J.: Is It X-Factor or H-Factor? Hair Transplant Forum Intl. 1994; 4(3): 10-11.
3. Seager, D.J.: Micrograft Size and Subsequent Survival. Dermatologic Surgery 1997; 23: 757-761.
4. Seager, D: Binocular Stereoscopic Dissection Microscopes: Should We Use Them?, Hair Transplant Forum Intl. 1996; 6(4): 2-5.
5. Greco, J., Kramer, R.: Presentation on Follow-up “Crush Study”. Intl. Soc. of Hair Restoration in Barcelona, Spain, October 15-19, 1997.
6. Cooley, J., Vogel, J.: Loss of the Dermal Papilla During Graft Placement: Another Cause of X-factor? Hair Transplant Forum 1997; 7(1): 20.
7. Gandelman, M.: Presentation on Micrograft Survival, ISHRS 5th Conf., Barcelona, Spain, October 15-19, 1997.
8. Limmer, B.L.: Micrograft Survival. IN: Stough, D.B., Haber, R.S., eds. Hair Replacement, Medical and Surgical: St. Louis, Mosby: 1996: (5) 147-9.
9. Goldman, B., The Effects of Hair Micrograft Surgery on Scalp Oxygen Levels. Presentation at 3rd ISHRS Meeting, Las Vegas, Nevada, 1995.
10. Klemp, P., Kansted, B.: Subcutaneous Blood Flow in Early Male Pattern Baldness. J. Dermotol 1989-92; 725.
11. Uebel, C.O.: Punctiform Technique with Micrografts – A New Method for Pattern Baldness Surgery. Free Paper Presented at Jornada Carioca Cirur Plast., Rio de Janeiro, Brazil, August, 1986.
12. Rose, P.: Presentation American Society of Hair Restoration Surgery in Orlando, FL, 1996.
13. Limmer BL: Elliptical donor stereoscopically assisted micrografting as an approach to further refinement in hair transplantation. Dermatol Surg 1994; 20: 789-793.
14. Bernstein RM, Rassman WR.: Dissecting microscope versus magnifying loupes with transillumination in the preparation of follicular unit grafts. A bilateral controlled study. Dermatologic Surgery 1998; 24: 875-880.
15. Cooley, J., Vogel, J.: Loss of the Dermal Papilla During Graft Placement: Another Cause of X-factor? Hair Transplant Forum 1997; 7(1): 20.
16. Rassman WR, Bernstein RM.: Rapid Fire Hair Implanter Carousel: A new surgical instrument for the automation of hair transplantation. Dermatol Surg 1998; 24: 623-627.
The Future in Instrumentation
James Arnold, MD
The imaginative minds of many surgeons have given us a glimpse of future developments in hair transplantation techniques and instrumentation. Efforts to simplify the work or to combine tedious steps include devices to automatically insert grafts, to cut donor tissue into multiple grafts in a single pass, and to lessen crush damage to grafts are surfacing.
The recipient site has recently received a new focus of interest among transplant surgeons reflecting a desire to lessen the traumatic effects, which occur from cutting hundreds of recipient sites in close proximity. New instrumentation has been developed with the intended goals of preserving the blood supply and minimizing scarring to the scalp. The concept of cutting recipient sites to an absolute minimum depth is based on the anatomical design of the vasculature supplying blood to the scalp1.
Montagna2 and others have demonstrated the primary distribution of blood to all areas of the scalp is through a network of arteries that lie beneath the dermis. This arterial system is strictly confined to the fatty, sub-dermal compartment with even the smallest arteriole rarely entering the dermal portion of the scalp. Small ascending vessels bring blood to the dermis, which is then distributed, through a vast network of capillaries, supplying the appendages of the dermis including the follicles. For the transplant surgeon, it is important to recognize that the basic arterial system lies totally beneath the dermis, while the dermis proper is supplied with capillaries alone. The importance of this information is related to the ability of the scalp to readily heal damaged capillaries. In contrast, the healing mechanisms for the underlying arterial network are more limited.
Capillaries have an outer wall of only a single cell in thickness and are replaced quickly if severed or damaged, via the process known as angiogenesis. Vessels of the arterial system, even the smallest arteriole, are compound structures with an endothelium lining, a muscular wall and an outer sheath. Re-canalization of arteries may occur, and existing anastomosis may expand, but neither response will be as efficient for providing blood to the scalp as the original arterial system. The concept of minimal depth recipient sites, therefore, is to limit cutting of the sites to the capillary dermis and avoid penetrating into the sub-dermal region where permanent compromise to blood supply may occur. In general, the capillary dermis is thick enough to allow recipient sites 3-4mm in depth without penetration into the sub-dermal compartment. Recipient sites 3-4mm deep are generally adequate in depth for transplant grafts.
There are several challenges for the surgeon in cutting minimal depth recipient sites. First, the depth of each site should extend the full 3-4mm, no less and no more. Second, the sites should be fully open at the base of the site to adequately accommodate the base of the graft.
In the past, lancet or spear-point shaped blades were popular among transplant surgeons for cutting recipient sites. Lancet blades, such as hypodermic needles, No-Kor needles, #11 scalpel blades, and spear point blades have a sharp point which facilitates penetration of the scalp. However, to open the deeper portion of the recipient site, lancet and spear-point blades must extend beyond the 3-4mm minimum, usually penetrating well into the territory of arterioles. A better design is a chisel-shaped blade, as the blade will cut an opening of uniform width along the entire length of the incision. In order for the flat, chisel-shaped blade to penetrate the scalp as easily as a pointed blade, the chisel must be extremely sharp. To control depth, limiting penetration 3 to 4mm, a “stop” can be used.
An instrument designed specifically for cutting minimal depth recipient site is the Min-de Knife TM (A to Z Surgical, Inc. San Jose, California). The instrument has a chisel blade sufficiently sharp to penetrate the scalp easily and the blade handle acts as a stop to control cutting depth to either 3mm, 3.25mm, 3.5mm or 4mm. Surgeons familiar with the instrument describe less bleeding occurring in the recipient area. As evidence of this, minimal depth incisions ooze from the dermal capillaries, but active bleeding from punctured arteries occurs less frequently than with lancet or spear-shape blades.
There is no doubt that many improvements will continue to evolve in the field of hair transplantation. While the basic techniques will remain the same, new methods and instruments will greatly simplify the process.
References
0. Arnold, J.: What’s new in techniques and instrumentation? American Academy of Dermatology Annual Meeting, February 27-March 4, 1998, Orlando, Florida. (AAD Audiotape 320A and B)
1. Montagna, W. Parakkal PF: The Structures and Function of the Skin. New York Academy Press 1956
Automated Graft Cutting
E. Antonio Mangubat, MD
The future of hair restoration surgery (HRS) lies in technology and automation. Contemporary HRS differs from most other cosmetic procedures in its high demand of skilled manpower, great dependence on non-physician assistants and prolonged procedure times. Our noted colleagues, including Marritt, Limmer, Seager, Bernstein, Rassman and others, have taught us the value of large numbers of small grafts in producing natural results. Rassman and Bernstein go on to advocate the exclusive use of follicular units in HRS.1
Although the HRS community universally recognizes the value of small grafts, there is still much controversy as to which methods yield the best results. Furthermore, managed care pressures have caused the influx of new HR surgeons creating intense market competition never before seen in HRS history. These forces have influenced the HRS community to become faster, more efficient yet strive for the best results.
The nature of contemporary HRS is repetitive tasks: 1) graft cutting and 2) graft placement. With the exception of a few individual patient variations, these tasks are virtually identical for all patients undergoing HRS. Repetitive tasks naturally lend themselves to automation. The focus of this section is the automation of graft cutting. The ideal graft cutter would produce large numbers of hair grafts with uniform size and shape and without follicular damage. The grafts would all be viable, easier to place and procedure times would be significantly reduced.
The use of impulsive force to significantly improve the quality of grafts processed with an automated graft cutter has been recently introduced into hair transplantation.2 Impulsive force is a physical concept defined by the equation:
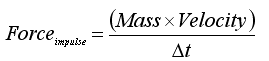
Where Δ is the duration of time during which the external force is applied.3 A cursory examination of the equation demonstrates that the smaller t for any given external momentum, the larger the impulsive force. Many common uses of impulsive forces are seen today including splitting wood with an ax, hitting a golf ball and a martial arts expert breaking bricks with a bare hand. The feasibility of these examples could not be explained without this physical concept. The latter example is most striking with a human hand applying a small external force for a very brief period of time producing impulsive forces large enough to break the brick material which is many times harder than human flesh. Prior graft preparation devices do not take advantage of this physical property and thus crush injury to the grafts may occur, especially if the blades have been dulled by prior use.
The impulse graft cutter consists of a stable base and three sets of parallel spacer bars, which hold the cutting blades rigidly in place. A series of long 1.0 mm donor strips are then taken with a multi-bladed knife. Harvesting high quality donor strips with a multibladed knife is greatly technique-dependent4 and is critical to successful graft cutting automation.
After trimming the excess fat and separating the donor strips, a strip is placed on the graft cutter carefully aligning the follicles with the cutting blades. A wooden tongue blade is used as a force spreader to cover the donor strip and hold it firmly in place while a rhinoplasty mallet is used to impart the impulsive force to the donor strip. Impulsive force then causes a clean shearing of the tissue with surprising little transection.
Follicular trauma can take many forms including transection, desiccation5,6 and prolonged tissue anoxia (time-out-body)7. Although graft cutting automation may potentially increase the risk of transection, it reduces the risks of desiccation and donor anoxia by the significant reduction in procedure times. In addition, automation decreases expenses, dependency on skilled staff, training requirements and ameliorates the disruption of staff turnover. The graft cutter places the responsibility of quality graft production back in the hands of the surgeon.
A major challenge that lies ahead is to make HRS more affordable. The expense of HRS lies in its time consuming nature. Technology will ultimately make HRS more affordable through efficiency, speed and reproducible results. This era in HRS is upon us.
References
0. Bernstein RM, Rassman WR, Szaniawski W, Halperin A: Follicular Transplantation. International Journal Of Aesthetic And Restorative Surgery 1995 3:119-132.
1. Mangubat EA. Impulsive Force: A New Method To Cut Grafts. International Journal of Cosmetic and Restorative Surgery 1998. 6:19-23.
2. Halliday D, Resnick R. “Collisions” In Fundamentals Of Physics, P 155-161, John Wiley & Sons, Inc., 1970
3. Arnold J: Pursuing The Perfect Strip: Harvesting Donor Strips With Minimal Hair Transection. International Journal of Aesthetic and Restorative Surgery 1995. 3:148-153.
4. Gandelman M: Light and Electron Microscopic Analysis of Injured Grafted Micrografts. Presented at the International Society of Hair Restoration Surgery, November 1997, Barcelona, Spain.
5. Gandelman M: Light And Electron Microscopic Analysis Of Injured Grafted Micrografts: Stage II. Presented at the International Society of Hair Restoration Surgery, September 1998, Washington, DC.
6. Limmer BL: Micrograft Survival In Hair Replacement: Surgical And Medical. Pg. 147-149. DB Stough And RS Haber Eds. Mosby. 1996
Commentary
The purpose of this section has been to introduce a new device that may significantly reduce the time and labor involved in graft dissection. In spite of its potential benefits, some of the co-authors have concerns about the automated graft cutting technique for the following reasons:
0. It is important to stress that the growth rate and clinical significance of transected hair follicles, either produced by the multi-bladed knife during harvesting, or from other aspects of the dissection process, has not been examined in well controlled studies and is, at the present time, highly controversial.
1. The successful use of the impulsive technique depends upon the generation of very thin strips using the multi-bladed knife. The experience of some of these authors has been that even with 3mm spacing, the multi-bladed knife can cause significant and unacceptable damage to the donor tissue. The very thin, 1 to 2 mm strips needed for this technique would be expected to cause even greater transection.
2. Dr. Kim reported an increased incidence of pseudocyst formation when transected follicles are implanted, especially the lower portion. At this time the clinical impact of this finding is unclear.
3. Avoiding follicular injury, especially transection, is in part dependent upon the ability of the relatively rigid follicular structures to be pushed aside during follicular dissection, as the cutting instrument passes through the surrounding dermis and subcutaneous fat. In theory, the rapid impact of the impulsive technique should decrease crush injury to grafts; however, it is also possible that by essentially “freezing” the tissue in place during the rapid impulsive force, follicular transection may actually increase. Well-controlled studies are needed in each of these areas.
Robert M. Bernstein, MD
Automating Graft Placement
With many physicians now converging on a single standard of quality (the follicular unit),1 the focus of hair transplantation is now moving to solve the problems addressing the cost of the procedure in terms of labor, time and money, and in the consistency and predictability of product. Newer techniques have evolved which move more hair in smaller and smaller units. More labor has been neeeded to deal with the increasing workload that these new techniques require. Labor intensive processes produce human variables, which lie at the heart of the problems defined herein. As the labor-intensive process is solved, costs will fall and quality of product will rise. The new standard of quality will then be in the reach of all physicians performing cosmetic hair restoration surgery.
Most surgical procedures utilize only one or two assistants as the surgeon performs the procedure. In hair restoration, the surgical procedure is largely performed by a large technical staff because it has become tedious, time consuming and monotonous. At times, the procedure becomes unmanageable and, in response to this, the surgeons have abdicated quality control to the technical staff doing the work. Results vary with the expertise of the staff and consistency becomes a hit-or-miss process. If a surgeon is fortunate to have good surgical management skills and a dedicated, loyal and stable staff, results will be more predictable. However, this should not be a precondition for quality surgery.
Automation lies at the heart of the solution for it can address problems of speed and quality, as well as allowing the surgeon to more easily regain control over the surgical process. The automation solution should accomplish the following goals:
0. Reduce surgical time to under 3 hours for a typical surgery.
1. Reduce labor to a surgeon and one or two assistants.
2. Reduce or eliminate many of the human variables associated with the surgical procedure.
3. Reduce the stress of the procedure on the surgeon and staff.
4. Reduce the training and skill requirements for the surgical staff.
5. Reduce graft damage from manipulation and/or drying.
6. Reduce the need for human quality control processes other than with the surgeons direct involvement.
7. Make the procedure safer for the patient so that it produces less trauma by reducing (a) medication, (b) anesthesia time, (c) wounding, (d) bleeding and (e) tissue exposure to hostile environmental factors.
To accomplish this, the various process involved in the hair transplantation process must be rethought and reengineered. For simplicity, the process can be divided into two phases: graft harvesting, and graft placement. Automation should be able to simplify the entire process either step-by-step or through some generally integrated solution. The current discussion addresses new instrumentation designed to facilitate the second phase of this process, namely graft placment.
Traditionally, sites (wounds) were created in the recipient area and grafts were placed into the pre-formed sites in a second step. In the traditional manual process, the grafts were handled a number of times, often exposed to drying at points along the way. During the placement phase, as in the dissection phase, graft damage and desiccation had to be minimized. Grafts were often grasped and squeezed repeatedly in the growth regions of the follicles, as staff members tried to implant them into the scalp.
Graft placement is a very delicate process and learning how to manually place the grafts in a competent and efficient manner, with minimal trauma, often requires months or years of experience. Preventing graft drying is equally important and ignoring these two important factors will negatively impact graft growth.
Automation devices available today include the Choi Hair Transplanter2 and the Rapid Fire Hair Implanter Carouseltm3. A third, the Hair Implanter Pen,4 is not yet commercially available.
Choi Hair Transplanter
The Choi instrument has, until recently, been the only device in use, which makes the recipient sites and places the grafts at the same time. However, it holds only one graft at a time and the loading process is laborious, time consuming and inefficient. It also works best with the more rigid, coarse Asian hair since it pushes (rather than pulls) the hair into the recipient site. Its advantage over the manual technique is that the manual skills for placing grafts is largely eliminated refocusing the technical skills to loading the Choi device by hand. However, for a well-trained technician, it can take longer to load the Choi device than it takes to place grafts by hand. Another advantage is that human variables are reduced during the placement process.
The Rapid Fire Hair Implanter CarouselTM
Like the Choi Device, the Carousel combines what was always a two step process into a one step process but is not limited to holding and delivering just a single graft. The Carousel uses a cartridge to hold 100 grafts in a controlled environment. The Choi Device employs a piston and pushes the grafts into the hole while the Carousel gently pulls the graft into the hole. The author believes that this mechanism is superior to the Choi mechanism as it protects the grafts from compression during the placement process thereby minimizing trauma. It is also more versatile, easily accommodating different shaft diameters. The Carousel should provide the following benefits:
0. Single Action for Graft Placement: Each recipient site is created and the graft is placed directly into the recipient site with a single mechanical action. In the majority of cases, the grafts will remain in place with no additional manipulation. In certain situations, the grafts may need a fine adjustment to keep them from lifting after insertion.
1. Less Bleeding: Bleeding is reduced in the majority of sites as immediate graft placement compresses many of the smaller blood vessels in the recipient site. The immediate insertion of the graft creates a tamponading effect on small open blood vessels.
2. Less Anesthesia Administered: The total anesthesia dosage is reduced commensurate with the shorter surgical time.
3. Less Graft Manipulation: Manipulation of the grafts during the placement process is virtually eliminated. The compression (squeezing) of the graft with forceps is no longer necessary for the placement process. This reduces the possibility of damage to the graft.
4. Controlled Graft Storage: There is no significant period in which the grafts are exposed to the atmosphere after they are prepared. A specially designed cartridge, which is the storage element for the grafts, keeps them moist in a saline (or lactated Ringer’s) droplet until they are placed into the recipient area. This process prevents the individual grafts from being touched after they are placed into the cartridge.
5. Less Emphasis for Quality Control Monitoring during Placing: Quality control emphasis involved with human variables are reduced.
6. Better Graft Accounting: Graft accounting is simplified because the count can be based on the slots filled within the cartridge and the number of times the device is loaded.
7. Less Staff Stress: The time consuming and laborious process of placing the grafts into holes or slits is reduced. Staff fatigue and eyestrain are minimized.
8. Appropriate Physician Focus: By reducing the tediousness of hair transplantation the surgeon is able to focus on the excision of the donor site, the design of the hairline, and the placement of the grafts.
9. Shorter Staff Training: The protracted training period required to teach graft placement is reduced. However, at least one member of the surgical team must be skilled in manual graft placement techniques in the event grafts are expelled during the placement process, or other situations that may require manual intervention.
10. Procedure Costs: An expedited surgical procedure frees facility capacity and the physician’s time to a significant degree. Because costs are a critical factor in the decision to elect transplantation as a solution to hair loss, decreased costs should reduce the threshold for the decision ot have surgery.
The Hair Implanter Pen
The Hair Implanter Pen was developed by Dr. Pascal Boudjema, a brilliant inventor in field of hair transplantation (he also invented the Calvitron). The Hair Implanter Pen utilizes a suction tip to grasp the end of a hair graft of any small size. The surgeon drags the graft into a pre-formed wound and then the suction is released along with the graft. As the mechanism does not grasp or squeeze the graft, there should be little or no trauma to the graft. The Hair Implanter Pen can increase the placing speed, thereby reducing the surgical time. This instrument should be commercially available in the near future.
The philosophy that “necessity is the mother of invention” will work to the benefit of both surgeon and patient. Newer automation technologies are inevitable in view of the problems in delivering today’s procedures involving the movement of large numbers of small grafts. We are moving to an integrated, technically based surgical procedure, less dependent upon many supportive staff, and less costly to deliver. Traditional surgeons’ art is the hub of cosmetic surgery and, as these new technologies evolve, the cosmetic surgeon will be allowed to focus upon that art in the same vein as the other traditional surgeries he/she performs regularly.
References
0. Bernstein RM, Rassman WR, Szaniawski W, Halperin A: Follicular Transplantation. International Journal of Aesthetic and Restorative Surgery 1995; 3: 119-132.
1. Choi YC, Kim JC: Single hair transplantation using Choi hair transplanter. J Dermatol Surg Oncol 1992; 18: 945-948.
2. Rassman WR, Bernstein RM.: Rapid Fire Hair Implanter Carousel: A new surgical instrument for the automation of hair transplantation. Dermatologic Surgery 1998; 24: 623-627.
3. Boudjema P: A new hair graft implanter: The hair implanter pen. Hair Transplant Forum Int 1988; 8(4): 1-4.
Advances in Laser Technology
Two major potential drawbacks of conventional “cold steel” created slit recipient sites that have been voiced are graft compression and decreased hair density when comparable amounts of donor material are transplanted into recipient sites in which bald tissue is not removed.1 With “cold steel”, whether needle, knife or other sharp instruments, the slit recipient site represents a stab wound during which the skin merely “pops open”, since recipient tissue is not removed. Laser assisted hair transplantation sought to address these shortcomings. Unfortunately, the “Laser” is often considered synonymous with the carbon dioxide (CO2) laser, which produces a significant thermal burn in the process of creating the recipient site. The advantage of the laser’s ability to rapidly create uniform recipient sites with less bleeding than conventional cold steel techniques has been overshadowed by the tissue injury it causes. This article will serve to introduce the reader to the Erbium: YAG Laser as a new means of creating recipient sites without the thermal consequences of CO2 lasers.
The carbon dioxide laser was first invented by Bell Laboratories in 1964 and offered the advantage of hemostasis while “cutting.” This laser emits light in the far infrared portion of the electromagnetic spectrum at 10,600 nm, a wavelength that is highly absorbed by water. Because approximately 70% to 80% of skin tissue is composed of water, this absorption is nonspecific and is the basis for the use of the CO2 laser as a cutting tool. Cutting is achieved by focusing the CO2 laser beam to a 0.1 – 0.2-mm spot size, resulting in a focal impact generating temperatures > 300 degrees Celsius at the immediate zone of injury.2 This results in various zones of thermal damage (burn wound) depending on the laser used. This burn wound creates a locus minoris resistensiae (an area of decreased resistance) in the skin, the skin “pops open” about this zone of thermal damage, and minimal amounts of bald tissue, corresponding to the tiny CO2 laser spot sizes employed, are removed. The spot size was 0.2 mm emitted by one CO2 laser in the creation of slits,1 and a 0.15mm spot size from another in which punctiform sites were created.3
Equally, if not more importantly, the use of the CO2 laser to create hair transplant recipient sites introduced additional, if not more, drawbacks than its use was intended to solve. The zone of thermal damage compromises blood flow and decreases the fibrin network that acts as a “biologic glue” to hold the grafts in place. Not surprisingly, some grafts were reported to “fall out”.4 This zone of thermal damage also compromises the proper oxygenation and nutritive flow to the grafts, thus compromising graft survival. Moreover, a zone of thermal necrosis during the acute phase of wound healing must first be removed by the body. This led to reports of impaired graft revascularization, increased inflammation and prolonged crusting of up to two weeks, delay onset of growth by up to eight weeks, as well as sparse growth and scarring. 3,5,6
Dr. Walter Unger, who has been studying the use of CO2 lasers in hair transplantation since 1993, has generated an impressive body of work that is admirable for the scientific approach brought to these studies. Because Dr. Unger came to the conclusion that the cosmetic results were not superior when the Sharplan laser was used, and superior cosmetic results were inconsistent with the Ultrapulse CO2 Laser (Coherent), this prominent hair transplant surgeon announced in early 1998 that he had abandoned the use of the CO2 laser for hair transplantation.7
Imagine a technological advance in which bald tissue is actually removed, yet there is no burn at the wound edges to compromise the building of the fibrin network, the “biological glue” that holds the transplants in place, or compromising blood flow that delivers the crucial oxygenation and nutritive flow essential for graft survival prior to graft revascularization. The Erbium: YAG Laser represents such a technological advance. This laser (Er:YAG) has an emission line at 2940nm coincident with the strongest absorption peak of water (at least ten times greater than CO2!). Equally, if not more importantly, the Erbium: YAG Laser’s wavelength is near a local collagen absorption peak at 3030nm. The result on impact is much greater precision of tissue removal, almost nonexistent thermal damage, and “actual removal” of tissue! For example, by using the Erbium:YAG Laser to create 1000 recipient sites we are also now performing 1000 “mini alopecia reductions”, without the longitudinal scars inherent to alopecia reduction surgery. With the 1mm spot size we have used over the past two years this represents 785mm2 of bald tissue removed (pie r2 = 3.14 x 0.5mm2 x 1000 = 785mm2) per 1000 grafts.
Graft compression is a separate issue we have all at one time or another encountered. When grafts are trimmed to fit into very small recipient sites (“skinny” grafts”), they may be more subject to injury and, in addition, some telogen hairs may be trimmed away.8 Importantly, one study showed up to 24%,9 and another presented by Dr. Beehner showed a loss of up to 33% of hairs,10 when such “skinny” as opposed to “chubby” grafts were prepared. “Skinny” grafts may also result in finer and frizzy hair growth as opposed to hair growing from “chubby” grafts.10 The advantage of bald tissue removal in not compressing such “chubby” grafts which also contain viable telogen hairs, as might be the case in a narrow slit, should be apparent.
In the initial studies using an Erbium:YAG Laser (Candela\Fotona) for hair transplantation, a total of 35 laser created recipient site scalp specimens were evaluated histologically by two “blinded” dermatopathologists. It was shown that, at the level of the lower reticular dermis and in the fat at the level at which a transplanted follicle would reside, the skin exhibited 0 to less than 10 microns of thermal damage as measured by an ocular micrometer on a microscope.11,12
An update on the original patient group who underwent hair transplantation with micro- and minigrafting with 2001 recipients sites created by Erbium:YAG Laser alone, and in combination with 1934 “cold steel” created slit recipient sites12 showed that bleeding from the Erbium:YAG Laser (a “cold laser”) created recipient sites was not a clinical problem and was easily controlled with tumescent local anesthesia. Oozing was similar between the Erbium:YAG Laser and cold steel created recipient sites and there was no apparent difference in the “take.” In addition, unlike the experience that has been reported with some CO2 laser created recipient sites, no grafts were known to “fall out.” Equally important, because of the similarity between the Erbium:YAG Laser and cold steel, there seemed to be no detectable compromise of oxygenation and nutritive flow to the grafts, as witnessed by similar “yields” in the growth of the grafts, and no infection or scarring has, as yet, been noted.
These observations are further underscored by the following: when the Erbium:YAG Laser was used as a “warm” laser to emulate the properties of a CO2 laser, we did note prolonged crusting by up to five days and a delay in the onset of hair growth by up to three weeks. An Er:YAG laser functions as a “warm” laser when very high pulse repetition rates are programmed into the Erbium:YAG Laser’s computer. These clinical observations cast strong doubt concerning any advantage of a recently introduced laser that combines CO2 and Erbium:YAG Laser beams into one. In fact, clinical experience suggests that the advantages of an Erbium Laser for hair transplantation would be, at least partially, abrogated utilizing such a device since it would limit the ability to create scalp recipient sites in which our goal is to preserve graft oxygenation and nutritive flow during one of the follicles’ most vulnerable periods.
Although the Erbium:YAG Laser used (Candela\Fotona) created slits, Erbium made slits are cumbersome and time-consuming to produce with the present technology. This is why circular recipient sites were the ones performed over the past two years in the majority of patients.
“Nirvana” in Erbium:YAG Laser hair transplantation has not yet been achieved and there have already been exciting advances since the initial group of patients was treated. Present power outputs from Erbium Lasers are less than ideal, unnecessarily slowing the procedure while leaving the door open to the inadvertent introduction of unnecessary thermal damage by laser users turning up the pulse repetition rate in an effort to compensate for the inadequate power output provided by some Erbium:YAG Laser manufacturers. A new 2500 mJ Erbium:YAG Laser seems to be clinically superior to the original 1000 mJ output.
It is important for the reader to realize that the beam profile of the laser beam emitted by some laser manufacturers is far from ideal. Indeed, some beam profiles have been reported to be in the shape of a “doughnut,” i.e. the energy is greatest at the sides with the central area possessing the lowest energy. This is not desired in a beam profile and leads to markedly increased zones of thermal damage at the wound edges, as has been noted by some investigators using such lasers.
There are a number of other advances that have recently been introduced. One manufacturer (Dornier Med Tech) already offers a telescopic handpiece capable of altering the beam diameter with a simple sliding bar built into the handpiece. Another laser manufacturer (ConBio) offers a variety of 0.5, 0.75 and 1mm spot sizes for use in hair transplantation. Most importantly, another manufacturer (Dornier Med Tech) is already in the final stages of releasing a “slit hand piece” truly capable of making slits of various dimensions quickly. This hand piece should be usable with a computer-generated scanner, as well as manually, to appropriately adapt to many transplant situations.
The ability of the new Erbium: YAG Laser to rapidly create uniform recipient sites, and its ability to create a slit while at the same time removing recipient tissue without causing significant thermal injury to the recipient bed, represents a significant advance over CO2 lasers. The Erbium:YAG Laser is an important new addition to the hair transplant surgeon’s armamentarium.
References
0. Unger W.P.: Laser Hair Transplantation IIII. Computer-assisted Laser Transplanting. Dermatol Surg 1995; 21: 1047-1055.
1. Nemeth A.J.: Lasers and Wound Healing. In: Nemeth A.J. (ed.): Dermatologic Clinics – Wound Healing. W.B. Saunders Co. Vol. II No. 4; 1993, pp 783-789.
2. Villnow M.M., Fenduni B: Update on Laser-assisted Hair Transplantation. Dermatol Surg 1998; 24: 749-754.
3. Bernstein R.J., Rassman W.: Laser Hair Transplantation: Is It Really State of the Art? Lasers Surg Med 1996; 19: 233-238.
4. Fitzpatrick R.E.: Laser Hair Transplantation. Tissue Effects of Laser Parameters. Dermatol Surg 1995; 21: 1042-1046.
5. Unger WP: Update On Lasers. Hair Transplant Forum Intl. 1997; 7(2): 19
6. Unger WP: Presentation on Laser and Controversial Hair Transplantation. Am Soc for Dermatol Surg in Portland, Oregon, May 13-17, 1998.
7. ISHRS Washington D.C. September 16-20, 1998
8. Seager D.J.: Micrograft Size and Subsequent Survival. Dermatol Surg 1997; 23: 757-761
9. Beehner M.: Two Research Studies on Follicular Unit Growth. ISHRS in Washington, D.C. September 16-20, 1998
10. Nemeth A.J., Miller I, Messina J.L., Glass F, Rehnke R.D: Erbium:YAG Laser-assisted Hair Transplantation: A Clinical and Histological Study. ISHRS in Barcelona, Spain, October 15-19, 1997.
11. Nemeth A.J.: Erbium Laser Hair Transplantation: Update and Present Status. ISHRS in Washington, D.C., September 16-20, 1998
Commentary
There is no doubt that the new Erbium: YAG Laser offers substantial improvement over traditional CO2 lasers in eliminating thermal injury. For those practitioners who use minigrafts and larger grafts containing multiple follicular units, the ability to rapidly create recipient slits and cause less graft compression, is a significant advantage. For those of us who use individual follicular units to keep the wound sizes to a minimum, the usefulness of the laser is less obvious. It is a concern to some of these authors that the wounding produced when recipient tissue is removed is not equivalent to a small “cold steel”slit. Well controlled studies are much needed to resolve this important issue.
Splitting Hairs
Not a hair transplant procedure is performed without at least implanting a few transected follicles. What happens if grafts with transected follicles are planted?
To answer this question, a study was performed in which excised skin from the human occipital scalp was cut by a surgical blade along the direction of hair growth. Intact individual human anagen hair follicles were isolated with a scalpel. Implants were prepared from follicles as follows:
0. The upper one-third and lower two-thirds of the follicle were obtained by horizontal section just below the pilo-sebaceous junction.
1. The upper and lower halves of the follicle were obtained from a transverse cut at the middle portion of the follicle
2. The upper two-thirds and lower one-third of the follicle were obtained from the transection of the follicle at the lower one-third of the follicle.
Both upper and lower follicle grafts were transplanted onto the forehead or leg. Histologic examinations were performed for each successive biopsy after grafting. Lower half follicles and intact (non-transected) follicles were cultured in Philpott’s medium1. Follicles were measured daily using inverted microscopes with a calibrated eyepiece graticule.
Eight months after grafting, 13 of the 20 grafted upper two-thirds, 25 of the 30 grafted lower two-thirds, 10 of the 25 grafted upper half, and 4 of the 15 lower half follicles have regenerated complete hair follicles. However, no hair follicles were regenerated from the grafted lower one-third and upper one-third follicles. The regenerated hairs from upper follicle implants were thinner than those from lower follicle implants.
A histologic examination showed that the regenerated hair follicle from the upper half follicle implant revealed the presence of a reformed small dermal papilla and a matrix. Lower half follicle implants reconstituted the complete hair follicle. Sebaceous glands were also completely regenerated. Some lower half grafts formed epithelial cysts.
Dissected hair follicles will grow and retain their morphology for 8 days in culture. Intact follicles show prominent naked shaft outgrowth. In contrast, the outer and inner root sheath grows concomitantly with the shaft in lower half follicles (1.5 mm after 8 days).
The results of this study showed that if the bulb containing the dermal papilla is removed, the hair will usually regrow. If the papilla is necessary for hair growth, how will hair regrow without it? A new papilla appears to be reformed from the connective tissue sheath2. However, the higher up in the follicle that transection occurs, the smaller will be the papilla that is reformed and hence the smaller and finer the hair is which is produced. The papilla determines the caliber of the hair shaft3. And if transection occurs above the midpoint of the follicle, a new papilla is not formed and hence no hair grows.
Thus, loss of the bulb region during graft dissection and placement is a clinically significant problem, which can contribute to incomplete and fine hair growth. However, this problem may be useful for the eyebrows, pubic hair, and female hairline reconstruction. In contrast to males, the female hairline is generally made up of fine vellus hairs that give it its “soft” character. One can successfully graft single hair follicles after removal of the bulb for the reconstruction of these regions. This technique can provide the best possible cosmetic result.
If the follicle is transected in its upper portion and is planted so that it is stranded in the dermis, cyst formation is likely4. Our results showed that some grafts formed epithelial cysts, but 25 of the 30 grafted lower two-thirds and 4 of 15 lower half follicles regenerated complete hair follicles. The sheath components grew out at the same rate as the shaft (1.5 mm for 8 days) in culture. This result suggests that the outer and inner root sheath grew out and could connect with epidermal invagination in vivo. If grafted hair follicles were located too deep, the re-grown sheath could not reach the epidermal layer. In this situation, the formation of an epidermal cyst is likely.
0. Philpott MP, Kealey T. Effects of EGF on the morphology and patterns of DNA synthesis in isolated human hair follicles. J Invest Dermatol 1994;102:186-191.
1. Kim JC, Choi YC. Regrowth of grafted human scalp hair after removal of the bulb. Dermatol Surg 1995;21:312-313.
2. Oliver RF. Whisker growth after removal of the dermal papilla and lengths of follicle in the hooded rat. J Embryol Exp Morphol 1966;15:331-347.
3. Cooley J. Rat whiskers, follicle transection, and hair transplantation. Hair Transplant Forum 1997;7(2):20-21.
The Future in Graft Storage
When performing hair transplantation procedures, it is of the utmost importance to try to obtain the maximum survival rate possible of transplanted micrografts. Although numerous factors (such as dehydration of grafts, trauma in handling with forceps, etc.) play a critical role in the graft survival, a “metabolic pre-conditioning” of hair grafts by means of dedicated storage mediums should have value in striving to enhance the survival rate of grafts when performing hair transplantation surgery.
Knowing the best way to preserve the grafts is particularly important with the advent of megasessions (involving the transplant of a large number of very small grafts), since a significant period of time may elapse between graft harvesting and their implantation in the recipient area. At present, it is generally recommended to preserve the grafts at a low temperature (1ºC – 4ºC), in order to enhance the survival rate of the grafted hairs1. Indeed, it is assumed that, as in other major transplant procedures, lowering the metabolism of the grafts by means of a reduction of their temperature may be of some utility for enhancing their survival rate. On the other hand, since the development of tissue preservation regimes has achieved great successes in the preservation of vital organs and grafts in the interval between harvest and re-plantation into the recipient area, other additional methods should be considered.
In this regard, for example, the role of oxygen free radicals in tissue ischemia has been well documented. Oxygen free radicals in ischemic tissue are generated from a number of different sources. The relative importance of these mechanisms is not known. It would follow, however, that the prevention of action of radicals produced by all of these pathways would be more effective in preventing tissue damage than by blocking the radicals produced by a solitary pathway2. Similarly, analysis of cellular metabolism in hypoxic conditions reveals not only an accumulation of cytotoxic metabolites, but also a gradual depletion of cellular energy stores3.
Increased intracellular use of high-energy adenosine triphosphate (ATP) and decreased mitochondrial regeneration of ATP occur under anaerobic conditions3-6. These changes lead to the deterioration of normal cellular processes and eventual cellular demise.
The above-mentioned considerations can be can applied to the field of hair transplantation surgery. Deferoxamine has been shown2 to be a potent, non-selective scavenger of oxygen free radicals, while exogenous ATP is thought to replenish depleted intracellular ATP, since several observations support the notion of intracellular uptake of extracellular ATP by ischemic cells. Indeed, addition of the exogenous high-energy cellular substrate adenosine triphosphate-magnesium chloride has been shown to substantially improve cellular preservation and tissue viability in ischemic conditions6.
In a recent study7, normal human occipital scalp samples were obtained, from ten healthy male patients, during routine excision of benign scalp lesions. Isolation of anagen hair follicles was achieved by stereo-microscopic dissection. A total of 200 anagen hair follicles (20 follicles per each sample) were obtained.
Follicles from each of the ten occipital scalp samples were thus randomly assigned to one of the following group: Group A (control; n=10 follicles per each scalp sample; total n=100 follicles), and Group B (experimental; n=10 follicles per each scalp sample; total n=100 follicles). While follicles from Group A were preserved, for five hours, in a Petri dish filled with isotonic saline, follicles from Group B were preserved, for five hours, in a Petri dish filled with saline containing adenosine triphosphate-magnesium chloride 0.1 µmol/ml and deferoxamine mesylate 15 mg/ml.
Immediately after the five-hour period, hair follicles from both Groups were stored in 500 ~l of Williams E medium with supplements as follows: 1% fetal calf serum, 10 ~g/ml transferrin, 10 ~g/ml insulin, 10 ng/ml sodium selenite, 10 ng/ml hydrocortisone, 100 U/ml penicillin, 100 ~g/ml streptomycin, 2.5 ~g/ml fungizone, and cultured for ten days8. Follicles were maintained free floating in individual wells of 24-well multi-well plates in an atmosphere of 37ºC, 5% CO2 – 95% air, and 100% humidity. This has permitted detailed measurements to be made on both the length and survival rates of the tested follicles.
The length of each follicle was measured at magnification x20 immediately following the five-hour test period and at the end of the ten-day culture period, using a calibrated microscope. Total follicle length was computed as the distance from the base of the bulb to the end of the shaft. Follicles which lost normal follicular architecture due to degeneration late in the culture period were computed as not survived. Histology was accomplished, at the end of the ten-day culture period.9 Most of the dissected follicles grew and retained their morphology for the whole ten-day period of culture.
A statistically significant difference was found between the survival rate (considered as preservation of normal follicular architecture and absence of degenerative signs) of follicles from the Control Group (87%) and Experimental Group (98%). Photographs taken of freshly isolated and maintained hair follicles showed that the increase in length over ten days was not associated with any disruption of hair follicle architecture. The length increase always occurred by the production of keratinized hair shaft. All the survived follicles produced a measurable shaft elongation, and no statistically significant differences were found between the growth rate of follicles from the Control and the Experimental groups. Histologic analysis demonstrated that the survived follicles from both Groups always maintained a normal histologic appearance, even after 10 days in culture.
The reported study addressed the question of whether an “enhanced” preservation solution, containing adenosine triphosphate-magnesium chloride and deferoxamine mesylate, is suitable for preservation of hair grafts, and whether this pharmacological/metabolic treatment may further enhance viability of transplanted micrografts. The survival rate of follicles from the control Group was consistent with that reported by Limmer10, storing the grafts in chilled isotonic saline at 4ºC. Conversely, our experimental data were indicative of a significant increase in the survival rate of hair micrografts pre-treated with adenosine triphosphate-magnesium chloride and deferoxamine mesylate.
In conclusion, it should be taken into account that the study discussed was just an in vitro study, and that the obtained results should be validated by further in vivo studies. Similarly, further studies need also to be undertaken in order to evaluate and compare other storage mediums. As hair transplant procedures continue to increase in length, metabolic pre-conditioning of hair grafts should play an important role in ensuring maximum follicular survival.
References
0. Fan J, Raposio E, Nordström REA: Minigraft preparation in surgical hair replacement. Scand J Plast Reconstr Hand Surg 1997; 31: 83-7.
1. Kohout M, Lepore A, Knight KR, et al.: Cool perfusion solutions for skin flaps: A new mixture of pharmacological agents which improves skin flap viability. Br J Plast Surg 1995; 48: 132-44.
2. Zimmerman TJ, Sasaki GH, Khattab S.: Improved ischemic island skin flap survival with continuous intraarterial infusion of adenosine triphosphate-magnesium chloride and superoxide dismutase: A rat model. Ann Plast Surg 1987; 18: 218-23.
3. Machiedo GW, Ghuman S, Rush BF, et al.: The effect of ATP-MgCl2 infusion on hepatic cell permeability and metabolism after hemorrhagic shock. Surgery 1981; 90: 328-32.
4. Maxild J.: Effect of externally added ATP and related compounds on active transport of p-aminohippurate and metabolism in cortical slices of rabbit kidney. Arch Int Physiol Biochim 1978; 86: 509-14.
5. Williams D, Reibel DK, Rovetto MJ.: ATP induced increase in ATP content in cultured myocardial cells. Fed Proc 1979; 38: 1389-93.
6. Raposio E, Cella A, Panarese P, Nordström REA, Santi PL.: Power-boosting the grafts in hair transplantation surgery: Evaluation of a new storage medium. Dermatol Surg (in press).
7. Raposio E, Filippi F, Levi G, Nordström REA, Santi PL.: Follicular bisection in hair transplantation surgery: An in vitro model. Plast Reconstr Surg 1998; 102 (in press).
8. Heidenhein M.: Uber die Maliorysche Bindegeweosfarbung mit Karmin und Azokarmin als Vorfarben. Ztsch F Wissensch Wikr 1915; 32: 361-4.
9. Limmer BL: Micrografts survival. In: Stough DB, ed. Hair Replacement: Surgical and Medical. St. Louis: Mosby Press 1996: 147-9.
The Future Of Wound Care
Wound healing involves a coordinated series of events involving specialized cells, polypeptide growth factors, proteinases and proteinase inhibitors, as well as nutritional factors. Initially after a wound is created, the body seals off or reduces blood flow into the area and neutrophils migrate to the site to secrete toxic superoxide and other highly reactive molecules to sterilize and induce a general inflammation.
In the next phase of healing, this inflammatory response is suppressed while macrophages and fibroblasts migrate into the injury to secrete specific growth factors and to begin the process of rebuilding tissue. The fibroblasts are stimulated to produce new collagen, proteoglycans and other extracellular matrix components, and new blood vessels are formed by capillary endothelial cells.
Non-viable tissue is removed, cell migration and new blood vessel formation are facilitated, and remodeling is accomplished through the action of specific proteinases (MMP or matix metalloproteinase). These proteinases are controlled through the action of specific inhibitors (TIMP or tissue inhibitor of metalloproteinase). Overall, many of the activities involved in wound healing have been shown to be controlled by specific growth factors secreted by cells involved in the process or liberated during the early stages of clot formation.
The Present
The modern wound healing theory is that optimal healing is based on maintaining a moist wound environment containing all the factors necessary for healing to proceed. The purpose of a wound dressing is to provide a protective environment for tissue healing. An optimal wound dressing should:1
• Maintain the proper humidity at the wound surface
• Control fluid (wound exudate)
• Be easy to apply and remove
• Allow air and water vapor to escape or enter
• Provide thermal insulation
• Provide a barrier to contamination
• Be non-toxic
• Conform to the wound surface
Modern wound care practices aim to optimize wound healing through the use of select nutritional and growth factors which bring an additional trait for an optimal wound dressing.
• Provide an enriched wound environment
Wound dressings used for acute and chronic wound care consist of a wide range of natural and synthetic materials.2,3 These dressings, used singly or in combinations, can provide many of the characteristics of optimum wound dressings in terms of protection of the wound from contamination and management of wound fluids.
In addition to the management of wound fluid and contamination, many modern wound dressings are developed to create an environment to optimize wound healing underneath the dressing. This is accomplished through the application of nutritional factors such as copper and zinc, botanicals such as aloe vera, and sterilants or odor control agents. For example, the combination of moisture management and nutrient copper supplementation in the form of the GraftCyte™ product line became available for cosmetic and dermatological wound care in 1997.
Tissue glues have received considerable interest as a speedy and safe equivalent to sutures. In the studies reported, their use resulted in significantly quicker closure with equivalent cosmetic results to traditional sutures9-13.
The importance of growth factors to wound healing has been discussed above and a number of factors (epidermal growth factor (EGF), transforming growth factor (TGF), and platelet derived growth factor (PDGF)) have been the subject of intensive investigation16-18. 1998 saw the introduction of a long awaited growth factor product in the wound care field. This was the first commercially available human growth factor (PDGF, Regranex® Gel, J&J) which was approved by the FDA for topical application to chronic wounds.
The Future
What might the future practice of wound care involve? Integration of the protective and fluid handling properties of modern synthetic dressings with direct application of factors to enhance the wound environment is the most likely scenario. These “interactive” dressings and treatments would allow the physician to intervene in a positive and stimulatory way in the healing process rather than just protecting the wound and letting nature take its course.
The recent approval of Regranex® Gel provides the first commercially available product to allow the practicing clinician to supplement the wound with a natural factor, which initiates and ultimately controls part of the process. The increasing availability of dressings and treatments which provide micronutrients and other factors will help provide the building blocks and cellular tools to further enhance wound healing.
One of the most exciting approaches to manipulation of the wound healing process involves the application of modern molecular biology techniques to the proven participation of growth factors in the healing process. There are several approaches to the addition of growth factors to damaged tissue involving genetic technology. This can be through either the genetic modification of cells and their subsequent introduction to the wound or direct in vivo introduction of the genes to the wound. Genetic methodology has the advantage that a constant, regulated source of the growth factors will result.
Skin is an ideal target organ for genetically modified cells, as the turn over of skin cells will eliminate the modified cells as part of the normal healing process. Genetically modified keratinocytes have been prepared by the insertion of a plasmid containing the TGF~1 or EGF gene. These genetically modified keratinocytes were shown to release high levels of the corresponding growth factor to wounds19,20. In a similar manner, genetically modified human keratinocytes over-expressing PDGF-AA were used as the epidermal part of a composite skin graft. The composite graft composed of the genetically modified keratinocytes performed significantly better than control grafts in an animal model21.
Direct transfection with genes coding for growth factors is also a promising therapy. Topical application or transfection with a plasmid containing the gene for aFGF has been shown to significantly increase the closure and breaking strength of experimental wound 22 as has particle transfer of the human EGF gene23.
The entry to the field of artificial human skin is another promising technology to be applied to dermatology and cosmetic surgery. These products hold the promise of an unlimited source of replacement skin (full or partial thickness) for reconstructive and cosmetic purposes. No longer will skin have to be recycled from another site nor will the clinician and patient have to wait for new skin to cover a defect. Additional advantages of these products is that they serve an additional role as “biological” bandages which possess many of the attributes of an optimum wound dressing. Of course, the obvious combination of genetically modified cellular components to artificial skin replacements brings together the ability to manipulate the healing process with the instant coverage provided by artificial skin.
All wound healing consists of an integrated sequence of events involving specialized cells, growth factors, proteinases and proteinase inhibitors, and nutritional factors. Current medical practice for wound care is based on maintaining a protected moist wound environment. Future directions for wound care will be based on the ability to manipulate the wound environment with micronutrients, growth factors, and living cells. These enhanced wound care strategies should lead to faster and more aesthetically pleasing results in our hair transplants, in both the donor and recipient areas.
References
0. Szycher M, Lee SJ.: Modern wound dressings: a systematic approach to wound healing. J Biomater Appl 1992; 7: 142-213.
1. Hanna JR, Giacopelli JA.: A Review of Wound Healing and Wound Dressing Products. The Journal of Foot and Ankle Surgery 1997; 36: 2-14.
2. Hess CT.: Wound Care Products: A Directory. Ostomy/Wound Management 1994; 40: 70-94.
3. Williams R, L, Armstrong D, G.: Wound healing . New modalities for a new millennium. Clinics In Podiatric Medicine And Surgery 1998; 15: 117-28.
4. Falanga V, Margolis D, Alvarez O, Auletta M, Maggiacomo F, Altman M, et al.: Rapid healing of venous ulcers and lack of clinical rejection with an allogeneic cultured human skin equivalent. Human Skin Equivalent Investigators Group [see comments]. Archives Of Dermatology 1998; 134: 293-300.
5. Sorensen J, C.: Living skin equivalents and their application in wound healing. Clinics In Podiatric Medicine And Surgery 1998; 15: 129-37.
6. Duinslaeger L, A, Verbeken G, Vanhalle S, Vanderkelen A.: Cultured allogeneic keratinocyte sheets accelerate healing compared to Op-site treatment of donor sites in burns. Journal Of Burn Care And Rehabilitation 1997; 18: 545-51.
7. Elson ML. Soft Tissue Augmentation with Allogeneic Human Tissue Matrix. Cosmetic Dermatology 1998; 11: 24-28.
8. Shigemitsu T, Majima Y.: The utilization of a biological adhesive for wound treatment: comparison of suture, self-sealing sutureless and cyanoacrylate closure in the tensile strength test. International Ophthalmology 1996; 20: 323-8.
9. Singer A, J, Hollander J, E, Valentine S, M, Turque T, W, McCuskey C, F, Quinn J, V.: Prospective, randomized, controlled trial of tissue adhesive (2-octylcyanoacrylate) vs standard wound closure techniques for laceration repair. Stony Brook Octylcyanoacrylate Study Group. Academic Emergency Medicine 1998; 5: 94-9.
10. Qureshi A, Drew P, J, Duthie G, S, Roberts A, C, Monson J, R.: n-Butyl cyanoacrylate adhesive for skin closure of abdominal wounds: preliminary results. Annals Of The Royal College Of Surgeons Of England 1997; 79: 414-5.
11. Quinn J, Wells G, Sutcliffe T, Jarmuske M, Maw J, Stiell I, et al.: A randomized trial comparing octylcyanoacrylate tissue adhesive and sutures in the management of lacerations [see comments]. JAMA 1997; 277: 1527-30.
12. Maw J, L, Quinn J, V, Wells G, A, Ducic Y, Odell P, F, Lamothe A, et al.: A prospective comparison of octylcyanoacrylate tissue adhesive and suture for the closure of head and neck incisions. Journal Of Otolaryngology 1997; 26: 26-30.
13. Mendez-Eastman S.: Negative pressure wound therapy. Plastic Surgical Nursing 1998; 18: 27-9, 33-7.
14. Genecov D, G, Schneider A, M, Morykwas M, J, Parker D, White W, L, Argenta L, C.: A controlled subatmospheric pressure dressing increases the rate of skin graft donor site reepithelialization. Annals Of Plastic Surgery 1998; 40: 219-25.
15. Robinson C, J.: Growth factors: therapeutic advances in wound healing. Annals Of Medicine 1993; 25: 535-8.
16. Falanga V.: Growth factors and wound healing. Dermatologic Clinics 1993; 11: 667-75.
17. Meyer-Ingold W.: Wound therapy: growth factors as agents to promote healing. Trends In Biotechnology 1993; 11: 387-92.
18. Ghahary A, Tredget E, E, Chang L, J, Scott P, G, Shen Q.: Genetically modified dermal keratinocytes express high levels of transforming growth factor-beta1. Journal Of Investigative Dermatology 1998; 110: 800-5.
19. Rosenthal F, M, Cao L, Tanczos E, Kopp J, Andree C, Stark G, B, et al.: Paracrine stimulation of keratinocytes in vitro and continuous delivery of epidermal growth factor to wounds in vivo by genetically modified fibroblasts transfected with a novel chimeric construct. In Vivo 1997; 11: 201-8.
20. Eming S, A, Medalie D, A, Tompkins R, G, Yarmush M, L, Morgan J, R.: Genetically modified human keratinocytes overexpressing PDGF-A enhance the performance of a composite skin graft. Human Gene Therapy 1998; 9: 529-39.
21. Sun L, Xu L, Chang H, Henry F, A, Miller R, M, Harmon J, M, et al.: Transfection with aFGF cDNA improves wound healing. Journal Of Investigative Dermatology 1997; 108: 313-8.
22. Andree C, Swain W, F, Page C, P, Macklin M, D, Slama J, Hatzis D, et al.: In vivo transfer and expression of a human epidermal growth factor gene accelerates wound repair. Proceedings Of The National Academy Of Sciences Of The United States Of America 1994; 91: 12188-92.
New Medicines as an Adjunct to Surgery
The search for new and effective agents to treat many different hair loss problems has been intensified by the increase in hair biology research taking place worldwide, from university-academic institutions to the pharmaceutical companies, all with a desire to profit from marketing such drugs which have been termed, “cosmeceuticals.” Millions of men and women of every race suffer from various forms of alopecia, the most common being AGA, where DHT aggravates genetically programmed scalp hair follicles, resulting in short, fine, miniaturized hairs.
There is a great need for drug therapies which specifically attack the metabolic pathways involved in the balding process. This section will describe the most recently approved products for AGA, along with some in clinical trial development that may be used either alone or as an adjunct to surgical interventions.1
5a-reductase Inhibitors
In this category there are the structural steroid competitive inhibitors that chemically resemble the substrate, testosterone, and bind to the active site of the enzyme so that DHT is not formed. Propecia (finasteride) has recently been FDA approved in the USA for men with AGA. Propecia is a specific 5a-reductase type II enzyme inhibitor, and does not bind to the AR, and is therefore not called an “anti-androgen”, but an androgen inhibitor.
The pharmokinetics of finasteride reveal that after a 1 mg dose serum concentration of DHT decreases by 65% in 24 hours. Serum concentrations of testosterone and estradiol increase about 15% but remain within normal limits. Prostate concentrations of testosterone increase about sixfold2. Finasteride is well absorbed in the GI tract, metabolized in the liver and excreted in urine and feces, with a half-life of 5 to 6 hours. Small nanogram levels of the drug are detectable in human semen, but are not thought to have any consequence in women who are exposed by sexual contact.
Three double-blind multi-center trials were conducted in men ages 18 to 41 years and the results of these trials have been presented as abstracts3,4. In combined results from two of the trials, 1553 men with mild to moderate male AGA of the vertex took finasteride 1 mg/day or a placebo orally for one year. After three months of treatment, the men who took finasteride were more satisfied with the appearance of their hair. At the end of 1 year, in a circle on the vertex scalp, a one inch diameter mean baseline hair count was 876. Patients who took the drug had an average of 107 more hairs than those who took the placebo. Hair counts were maintained for up to 24 months in the men who continued to take the drug4. A third study in 326 men with mild to moderate frontal hair loss found that after one year, finasteride treated men had statistically significantly higher hair counts in their frontal scalp. Approximately 50% of treated men and 30% of those who took placebo thought the appearance of their hair had improved. Hair regrowth was not reported in older men taking 5 mg finasteride (Proscar), perhaps because it was not indicated in those trials to make observations on the scalp.
Adverse events described with 5 mg finasteride (Proscar) in a small percent of older men were loss of: libido and erection, ejaculatory dysfunction, hypersensitivity reactions, gynecomastia and severe myopathy2. Finasteride 1mg causes a 30 to 50% decrease in prostate specific antigen (PSA) in clinical trials in men 18 to 41 years old. The decreased libido, erectile dysfunction or a decreased volume of ejaculate have been reported in less than 2% of patients, which in reality is between 0.5% to 1% when compared against placebo and these effects are reversible when the drug is discontinued.
Finasteride has teratogenic effects in animals when taken in high doses, causing genitourinary abnormalities in male offspring. The concentration of the drug in semen of men who took 1 mg/day was much lower than the concentration associated with teratogenic effects in monkeys. Merck warns that women who are, or who may become pregnant should not have exposure to finasteride orally or by exposure of handling crushed or broken tablets, due to possible adverse effects on the male fetus. Finasteride is not indicated for use in women at the present time. There have been no published benefits for the use of finasteride in post-menopausal women thus far.
Dutasteride (GI198745, GlaxoWellcome) is a dual 5a-reductase inhibitor blocking both type I and II isoenzymes, which is currently in clinical trial studies around the USA for males with AGA. It is structurally similar to the parent structure of finasteride, maintaining the 4-aza structure of the steroid nucleus. However on the 21-carbon position is a tri-fluorophenyl group which renders the molecule to be electronegative and perhaps gives it greater affinity for both the type I and II isoenzyme forms of 5a-reductase5. Therefore, this drug is similar to finasteride in structure, but different in that it competitively inhibits both forms of the 5a-reductase type I and II isoenzymes, whereas, finasteride is just specific for inhibiting type II 5a-reductase. Dutasteride is known to inhibit >90% serum DHT levels in 24 hours after oral administration, and because of this greater ability to inhibit DHT, it is thought that it may be more effective in promoting hair growth, as well as treating acne. The results of clinical trials are pending.
Vascular/Angiogenic Related Compounds
Regaine/Rogaine (minoxidil) 2% has been used worldwide for over 10 years, and is now over the counter (OTC) in the USA. Most recently, Extra Strength 5% Rogaine has hit the OTC shelves in the USA, with approval in November 1997. Pharmacia-Upjohn has sole rights to being the only manufacturer for the next 3 years for this new version of minoxidil, which is indicated only for men.
Despite lack of understanding of the distinct mechanism of action, in women it has been shown to increase the non-vellus hairs when using it for 32 weeks or more2. One potential drawback to minoxidil therapy is that spontaneous reversal to the pretreatment state that can be expected one to three months after cessation of therapy, indicating minoxidil has a direct effect on the hair follicle, sensitizing it and making it dependent on the drug for future growth. The mechanism of action although still unclear, seems to open potassium channels and increases proliferation and differentiation of epithelial cells in the hair shaft2.
Serum concentrations after topical application of 2% minoxidil, used twice a day are generally about 5% of those with oral minoxidil, and with the 5% solution they are about 10% of those treated with the oral drug. Minoxidil is metabolized in the liver and excreted in the urine.
With respect to effectiveness, four unpublished 32 to 48 week studies presented to the FDA compared the effects of placebo, 2% minoxidil and 5% minoxidil by counting the net gain in hairs in 1 cm2 areas of the scalp. As described2, two studies in women did not find statistically significant differences between 2% and 5% minoxidil. A 32-week study in men found that the mean increase from baseline in hairs/cm2 was 5 with placebo, 30 with 2% minoxidil and 39 with 5% minoxidil. A 48-week study in men found a mean increase in hairs/cm2 of 3.9 with placebo, 12.7 with 2% minoxidil and 18.5 with 5% minoxidil. Previous studies have shown that when the drug is stopped, all of the newly regrown hair falls out6. Despite these reports the new advertisements claim 45% more effective hair growth than regular strength 2%, with regrowth occurring as early as 2 months with overall 5 times more hair regrowth than placebo, with no major safety concerns.
Most physicians and lay people who have been using minoxidil for many years are not concerned about safety aspects, since most feel it to be a very safe product. Concerns are more on the “effectiveness” of the product in promoting and maintaining hair growth. The new 5% Extra Strength brings about a new glimmer of hope in showing improved hair growth for individuals that may not have seen results with 2% minoxidil.
Adverse effects noted with oral minoxidil include tachycardia, angina pectoris and fluid retention. When taken orally during pregnancy, minoxidil has been associated with hypertrichosis of the fetus and congenital anomalies. One double blind study in 35 balding men found that topical use of 2% minoxidil caused small but statistically significant increases in left ventricular end-diastolic volume, cardiac output and left ventricular mass2. Infrequently, dizziness and tachycardia have been reported with 2% solution, with advice given to patients to reduce frequency of application which helps in eliminating these side effects. Local irritation, itching, dryness and erythema may occur with the use of topical minoxidil, most likely due to the vehicle formulation of alcohol and propylene glycol.
The conclusion on minoxidil 5% and 2% solutions are that they can produce a modest increase in hair on scalps of young men with mild to moderate hair loss, with continuous application for years to maintain the effect. Questions as to use of 5% Extra Strength in women are being posed, with some clinicians already giving this to young women with early hair loss, even though it is only indicated by the manufacturer Pharmacia-Upjohn for use in men.
Many patients may be asking their physicians now and in the future about using both topical Extra Strength 5% Rogaine along with oral 1 mg Propecia, which many believe may be beneficial working together synergistically, however further human clinical trials are needed to verify this, since the only previous study was performed in the Macaque monkey model, which did show benefit when used together7.
Although other vasodilatory/angiogenic related compounds are progressing through the development pipeline1, it is difficult to ascertain their effectiveness until human clinical trials are performed. Many compounds that mimic minoxidil in vasodilatory properties, fail to show the same results, hence there may still be a unique mode of action about this compound that is yet to be fully uncovered. Unpublished investigations have suggested minoxidil to have oxidative-reductive potential to facilitate cofactor reactions necessary in side chain steps for hair follicle growth, as well as other suggestions of stimulating some of the keratin genes of hair matrix cells for synthesis of hair shaft keratins, producing thin, fine, indeterminate hairs, often seen with continued use of minoxidil.
Medications as an Adjuvent to Surgery
Currently available medications should prove to be useful adjuncts to surgical hair restoration for a number of reasons.
• Medications work best in the younger patient who may not yet be a candidate for hair transplantation.
• Medications are less effective in the front part of the scalp, the area where surgical hair restoration can offer the greatest cosmetic improvement.
• Medications can regrow, or stabilize hair loss, in the back part of the scalp where hair transplantation may not always be indicated.
• Although medications are of little use in patients who have extensive baldness, these patients are often ideal candidates for hair transplantation.
• If medications are shown to be safe and effective in the long-term, they will allow the hair restoration surgeon the ability to creat more density in the needed areas (such as the frontal hairline), since keeping reserves for future hair loss will be less of a concern.
With different medical options now available, patients must be educated as to their choices, advised as to how these agents work, educated to the fact that these drugs need to be used continuously throughout life in order to be of benefit, and be aware that long-term risks are not yet known. Most importantly, they must be provided with realistic expectations regarding the inability, at the present time, for medications to regrow substantial amounts of hair in the average patient. As newer, more effective agents are developed, it is certain that they will play a more expanded role in the hair restoration process.
References
0. Sawaya ME: Alopecia – the search for novel agents continues. Expert Opinion in Therapeutic Patents 7(8):859-872, 1997.
1. Abramowicz M (Ed): Propecia and Rogaine Extra Strength for alopecia, In: The Medical Letter 40(1021):25-27, 1998.
2. Kaufman K: Clinical studies on effects of oral finasteride, a type 2 5a-reductase inhibitor on scalp hair in men with male pattern baldness. Proceedings from First Tricontinental Meeting of Hair Research Societies 1995.
3. Kaufman K: Updates of clinical phase III trial data and results presented at the American Academy Dermatology Meeting, San Francisco, CA, March 1997, as well as the World Congress Meeting, Australia, June 1997.
4. Bramson HN, Hermann D, Batchelor KW, ET AL: The unique preclinical characteristics of GG745, a potent dual inhibitor of 5AR. J Pharmacotics and Experimental Therapy, 1997.
5. Kidwai BJ, George M: Hair loss and minoxidil withdrawal. Lancet 340:609-610, 1992.
6. Diani AR, Mulholland MJ, Shull KL, et al: Hair growth effects of oral administration of finasteride, a steroid 5-alpha reductase inhibitor, alone and in combination with topical minoxidil in the balding stumptail macaque. J Clin Endocrinol Metab 74(2):345-350, 1992.
Cloning
In August 1967, R.F. Oliver published a paper titled The Experimental Induction of Whisker Growth in the Hooded Rat by Implantation of Dermal Papillae1, in which he described – as the title implies – the growth of a whisker hair from the implantation of only dermal papillae cells. Prior to this paper he had demonstrated that implantation of the lower third of the vibrissa follicle wall would produce a hair, but the implantation of the upper two thirds of the follicle would not2. The August 1967 report took the study one step further by showing that even the lower third of the follicle was unnecessary and that dermal papilla cells alone were sufficient to produce hair growth. Other authors have shown that there is an inductive role of the dermal papilla during the ontogeny of pillage and vibrisae follicles3.
The implications of these results in rats, with regard to human subjects with Male Pattern Baldness (MPB), can easily be discerned. If we were able to culture human dermal papilla cells (DPC) from a “permanent” hair-bearing donor area in a patient with MPB, and implant them into the bald area of the same patient and grow hair, we could potentially have an unlimited supply of donor hair with which to treat that patient. Thus any hair density and coverage would be made possible in any patient after harvesting a single small piece of donor skin.
With cloning, those portions of hair transplant surgery during which donor tissue is anesthetized, excised, and divided into grafts would no longer be necessary. In addition, the handling of donor tissue during sectioning and insertion into the recipient area that can potentially result in decreased hair survival, could be reduced or eliminated. In effect, many of the time consuming, skill dependent, potentially damaging and costly aspects of hair transplanting could be avoided if a technique for successful culturing and transplanting of DPC could be perfected. Lastly, the need for more invasive surgical procedures such as Alopecia Reduction, Scalp Expansion and Extension, and scalp flaps could be eliminated.
In the summer of 1997, Professor Dan Sauder, chief of the Department of Dermatology at the University of Toronto, Gulnar M. Shivji, Shabana Shahid, and Dr. Walter Unger, met and decided on a series of steps for the investigation of cloning in humans. Their work is summarized as follows:
1. Identify and Grow Hair Dermal Papilla Cells (DPC) in culture.
2. Perfect techniques to grow large numbers of DPC quickly i.e. ideal culture media and nutrients.
3. Introduce the DPC into athymic mice or SCID mice and grow hair.
4. Introduce DPC into the human donor skin and grow hair.
5. Devise optimal methods of introduction of DPC into the human donor.
6. Learn how to grow hair with uniform density, with growth in the correct direction and at the correct angle.
Although it was initially thought that accomplishing the first two steps would be relatively easy, nearly a year was spent completing that task. Table #1 outlines the process of identification and characterization of human DPC. The first step in obtaining DPC involves dissecting the dermal papilla from a small graft containing one or more hairs in the anagen phase of growth. The dermal papilla is carefully excised from the remaining hair structure and placed into a tissue culture dish containing medium. These cells may or may not be DPC, and one must learn how to distinguish between them and epithelial cells or endothelial cells, which inhabit the same area of the follicle. Once the cells grow in culture, their true identity as DPC are confirmed with three different types of tests. The first is a morphological comparison, the second is confirmation using antibody stains that specifically react with components of only DPC, and the third is electron microscopy in which the ultrastructure is studied and confirmed to be that of DPC rather than epithelial or endothelial cells.
It should be clear from the above, that the task is not as simple as many of us clinicians would believe. Cells can be incorrectly thought to be DPC, primary cultures are easily contaminated by bacteria, the cells of some patients seem to grow far more easily than others, and finally propagating cells by sub-culturing is sometimes successful and sometimes not. In addition, the morphologic characteristics of DPC tend to change as their passage numbers are increased and they also will probably ultimately prove to be less effective in producing hair when they are finally introduced into the test animals.
Despite the above, we are very positive that we can now get millions of functioning DPC cells from one cell in most patients and that we will probably be able to do this successfully in all patients with further development of our expertise. Studies on athymic mice will begin as soon as approval has been received from the ethics committee of the University of Toronto.
After further refining laboratory studies, we will eventually be ready to introduce the cells into the human donor from which they came. Should we be successful in growing hair, the remaining two stages – that of devising the optimal methods of introducing the DPC into the donor and learning how to grow these hairs with a uniform density, in the correct direction and at the correct angle remain as large clinical barriers we have to overcome.
References
1. J. Embriol: Exp. Morph. August 1967; 8(1): 43-51.
2. Oliver R.F.: Ectopic Regeneration of Whiskers in the Hooded Rat from Implanted Lengths of Vibrissa Follicle Wall. J. Embriol. Exp. Morph. 1967; 17: 27-34.
3. Embryonic Papillae, Edward J. Kollar: The Induction of Hair Follicles. The
Journal of Investigative Dermatology 1970; 55(6): 374-378.
Genetic Therapies for Androgenetic Alopecia
Imagine that in your practice in the year 2010, in the corner of the room stands a futuristic incubator full of tiny plastic dishes with living, growing human dermal papilla cells from anonymous donors with a whole spectrum of different hair colors and textures. Each of the dermal papilla micro-cultures was already genetically engineered with an ensemble of genes known to control the growth, cycling and color of the hair for a lifetime. In addition, the grafts were engineered to be universal donors, by de-programming their immune imprint and re-setting the imprint to that of the recipient. Your patient enters the room, prepared for today’s “treatment”, which involves filling your gene gun with papilla micro-cultures, and permanently implanting engineered cells into the scalp. These magical cells have the ability to perfectly recapitulate the individual’s entire genetic hair program, for the rest of the recipient’s life. Should your patient need a little fine-tuning, no problem – a topically applied growth factor cream to selectively activate and silence the artificial ensemble of genes will do the trick.
Sounds like science fiction? Think again. For the first time ever, Dermatologic scientists have begun to take aim at a genetic understanding of androgenetic alopecia (AGA), and if the current pace keeps up, we will have a good handle on the genes governing the human hair cycle in the next few years. The first human gene known to be involved in control of hair cycle regulation is called “hairless”. It was cloned in our laboratories earlier this year, and proven to be the genetic cause of a simple form of inherited atrichia1. We anticipate it will be the first of many genes which work together to drive the hair cycle. With discoveries like this, we will soon be in a position to design rational cell-based and gene-based therapies for hair loss, in contrast to the currently available treatments. If all of this sounds just around the corner, however, we must pause and realize that the identification of susceptibility genes for AGA is still a few years away, not to mention the translation of those genes into a meaningful and rational cellular or genetic therapy.
If we look back in history, it is clear that we are poised for a genetic revolution in our understanding of hair growth at its most fundamental level. Here, we outline our current thinking of AGA as a complex genetic trait, and discuss our strategies to identify susceptibility genes for AGA. It is never too early to imagine what the practice of dermatology will be like in the year 2010, and we believe the future holds great promise for the application of this knowledge in the clinical setting.
The Historical Perspective
Correspondence shows that John D. Rockefeller Sr.’s problem with hair loss began in 1886 at the age of forty-seven, when he began ordering bottles of hair restorative. In 1893, his alopecia worsened as he struggled with digestive problems and fretted over the University of Chicago’s finances. Alopecia totalis, or the total loss of body hair, has been attributed to many causes, ranging from genetic factors to severe stress, but remarkably little is known for certain. In March of 1901, his symptoms worsened markedly, his moustache began to fall out, and all the hair on his body had followed by August.
The change in his appearance was startling. He suddenly looked old, puffy, stooped, and all but unrecognizable. He seemed to age a generation. Without hair, his facial imperfections grew more pronounced. His skin appeared parchment dry, his lips too thin, his head large and bumpy. Soon after losing his hair, Rockefeller went to a dinner thrown by J.P. Morgan (one of the few public dinners he ever attended) and sat down next to a mystified Charles Schwab, the new president of U.S. Steel. “I see you don’t know me, Charley,” said Rockefeller.
“I am Mr. Rockefeller”.
Coming on the eve of the muckraking era, Rockefeller’s alopecia had a devastating effect on his image: It made him look like a hairless ogre, stripped of all youth, warmth and attractiveness, and this played powerfully on people’s imaginations. For a time, he wore a black skullcap, giving him the impressively gaunt physiognomy of a Renaissance prelate. One French writer wrote “under his silk skull-cap he seems like an old monk of the inquisition such as one sees in the Spanish picture galleries.”
The alopecia dealt a blow to Rockefeller’s morale. The psychological effect is crushing for most people, and he dabbled restlessly in remedies. His physician started him on a hair-restoration regimen in which he took phosphorus six days a week and sulfur on the seventh. When such remedies failed, Rockefeller decided to buy a wig. Self-conscious at first and reluctant to wear it, he tested it one Sunday at the Euclid Avenue Baptist Church. Before the service, he stood in the pastor’s office, nervously adjusting it and telling a listener what an ordeal it would be to wear it in the church. When the wig met with a good reception, he was almost boyishly elated. Soon, he grew to love this wig, telling daughter Edith, “I sleep in it and play golf, and I am surprised that I went so long without it, and think I made a great mistake in doing so.”
He became so fond of wigs that he started to wear rotating wigs of different lengths to give the impression of his hair growing and then being cut. He even had wigs styled for different occasions: golf, church, short walks, and so on. For all his wealth, however, Rockefeller could never find the ideal wig. Starting out with a fashionable wig maker on the Rue Castiglione in Paris, he grew disillusioned when springs in the framework pushed up through the hair. He then switched to a Cleveland wig maker whose product had another maddening defect: The foundation fabric would shrink, making the wig suddenly slide across his bald pate.
What God had taken away, it seems, could never be perfectly restored.
– excerpted from Titan by Ron Chernow, 19982
This passage from a recent biography of John D. Rockefeller, Sr. captures the essence of living with alopecia from the standpoint of the patient2. We empathize with his fruitless searching for remedies, we are humbled and moved by the erosion of his self-esteem, we are chilled by the description of his ghoulish appearance, we cheer when his wig is met with approval in the church, we laugh and cry with him at the tales of him searching the globe for the perfect hairpiece, and we are intrigued by the observation revealed later in the book that his mother suffered from the same condition.
Equally chilling, however, is the observation that in the 100 years since Rockefeller’s alopecia began, at least three things remain unchanged: the devastation of its victims, the absence of a cure, and importantly, the profound lack of scientific knowledge about its pathogenesis.
A century later, with the advent of modern genomics, we stand uniquely poised to bring an end to all three.
The Answers are in Our Genes
Alopecia areata (AA) affects approximately 1.7% of the United States population, or approximately 4.6 million individuals, including males and females of all ages and ethnic groups3,4. A second, more common form of hair loss, androgenetic alopecia (AGA), also known as male pattern baldness, affects 50% of all males over age 50 in the United States, and a large percentage of women, generating a combined figure of nearly 40 million individuals who suffer from some form of inherited hair loss5-7, most of whom spend billions of dollars each year on ill-designed treatments, not cures, to combat their disease. While it is clear that AGA is a hormonally-modulated phenotype, it is clearly not due to single gene mutations in a single gene, such as the 5?-reductase genes8,9, and it fits the criteria to be modeled and analyzed as a primary polygenic disorder for the first time.
In men with AGA, the reported effects of hair loss are: considerable preoccupation, stress, anxiety, and coping efforts10,11. These effects are more pronounced in men with extensive hair loss, and among younger, single men, and those with an early onset of hair loss. Relative to control subjects, balding men had less body-image satisfaction yet were comparable in other personality indexes. Although most men regard hair loss to be an unwanted and distressing experience that damages their body image, many balding men actively cope and generally retain the integrity of their personality functioning10,11.
In contrast, similar research revealed strikingly deleterious psychosocial effects of AGA in women12,13. The vast majority reported that their hair loss engendered considerable anxious preoccupation, helplessness and feelings of diminished attractiveness. Women worried that others would notice their hair loss and that the condition would progress and become more socially apparent. Such stresses also gave rise to active coping efforts. Many have sought information and selective social support, struggled to control their disruptive negative thoughts and feelings about their condition, tried to conceal their hair loss with altered hairstyles, and engaged in compensatory grooming activities to try to restore their body-image integrity. AGA was clearly more disturbing to affected women than a control group of women with less visible cutaneous disorders, and the psychological impact of androgenetic alopecia was found to be two-fold higher in women than in men12,13.
The authors of these studies emphasize that physicians should recognize that the pathology of AGA goes well beyond the physical aspects of hair loss and growth10-13. As has been observed in other appearance-altering conditions, they noted that patients’ psychological reactions to hair loss were less related to the clinician’s ratings than to the patient’s own perceptions of the extent of their hair loss. Even in patients with minimal hair loss, that loss carries significant emotional and psychological meaning that the physician should not minimize. The losses pertain not only to hair, but profoundly impact the quality of life, the ability to function in society, the preservation of self-esteem, and can lead to psychological disturbances as profound as suicide. One recent statistic quoted that 45% of men with AGA would sacrifice 5 years of their life span for a full head of hair14.
The two prescription drugs currently available for AGA are largely unsatisfactory, and were serendipitously found to grow hair in men taking these drugs for other indications, including hypertension and prostate hypertrophy. Astonishingly, neither of these treatments arose from primary research on the genetic mechanisms driving the hair cycle. Similarly, a rationally designed treatment for AA based on a fundamental understanding of the disease process is sorely lacking. Desperate patients spend tens of millions of dollars a year for remedies, yet all of this is in vain in the absence of a basic knowledge of hair loss as a genetically controlled process. Only genetic therapies targeting the hair cycle itself will lead to a cure for hair loss, in contrast to the available treatments which ineptly address only downstream effects.
For the first time in history, we are in a position to address these devastating dermatologic diseases from their foundation, as complex genetic disorders. With the promise of completion of the Human Genome Project in 2005, these studies are timely in a way not possible up to now, and will likely lead to the identification of candidate susceptibility genes by the end of the five years15.
We present a strategy for the identification of candidate genes in the control of the hair cycle, starting with simple Mendelian forms of inherited alopecia, and expanding into genome-wide linkage studies in complex genetic diseases including AGA. These studies will pinpoint candidate genes for the first time, lead to an understanding of the interactions of these genes with each other and with other variables such as the immune system and hormonal differences, and ultimately illuminate potential rational therapeutic targets for the future.
Male Pattern Baldness as a Complex Trait Disorder
Mendelian forms of diseases such as inherited alopecias are rare, clear cut, black-and-white examples of disease segregation: a family member can either be affected or unaffected. Mendelian traits ‘run in families’ (segregate) in clear and reproducible patterns, usually autosomal recessive or dominant.
The term “complex trait”, in contrast, refers to a more common genetic disorder that appears as a spectrum of shades of gray, rather than black and white. This term is used to describe any phenotype that does not exhibit classic Mendelian recessive or dominant inheritance attributable to a single gene locus15-18. The genes contributing to the phenotype can exist in such a way that they confer either additive or multiplicative degrees of susceptibility to developing disease15. The genetic analysis of complex non-Mendelian traits is most efficiently and powerfully studied using a technique known as Affected Sib Pair (ASP) analysis19-25. For two parents considered jointly, the two offspring can either share 0, 1, or 2 alleles with an average of 1 under no linkage. Testing for linkage amounts to determining whether the observed number of alleles shared over many ASPs is significantly higher than expected by chance, a phenomenon known as IBD, or identity by descent.
Once a complex trait has been mapped to several susceptibility loci, the formidable task of identifying the responsible gene still remains. Up to now, this has proven the greatest challenge in complex trait analysis, however, the timeliness of this proposal is reflected in the promise of the Human Genome Project to make a tremendous contribution to the positional cloning of complex traits by eventually providing a complete database of all genes in a given region15. Upon its completion near the year 2004, the Human Genome Project will facilitate the rapid and systematic evaluation of candidate genes in inherited alopecias.
The starting point for the genetic dissection of complex traits lies in the demonstration that genes are indeed an important part of whether an individual is at increased risk for disease predisposition. The observation that a trait ‘runs in families’ (aggregates) in an ill-defined pattern is not sufficient evidence to make the a priori assumption that its etiology is genetic, since families may share predisposing environments as well as genes. With this starting point in mind, we present the following arguments in favor of polygenic inheritance model in AGA.
Is Androgenetic Alopecia Genetic?
It has been widely cited in the Dermatologic literature that AGA is caused by a single autosomal dominant gene with reduced penetrance in women3,6,12,26. Amazingly, there exists only a single extensive family study published by Dorothy Osborn in 1916, where she studied the pattern of hair growth in 22 families and concluded that AGA is an autosomal dominant phenotype in men and a recessive phenotype in women27. She believed that a single gene dosage (Bb) causes AGA in men, where a double dose (BB) would be necessary in women. She gave no details regarding the methods of her examination, and weakened the validity of her data by stating that she had simply omitted the symptom-free women in her pedigrees27,28. Nonetheless, her conclusions were adopted, cited, and re-cited until the present, by many writers in both the dermatologic and genetic literature.
While careful family studies of AGA are still lacking, in 1984, Küster and Happle reconsidered the autosomal dominant model of AGA, and put forth several arguments against a simple mode of Mendelian inheritance. What they did, in fact, was to meticulously define AGA as a classic example of a polygenic trait28.
First Argument: Prevalance of the AGA Trait
It is common knowledge that AGA is widespread in all populations, yet it is difficult to determine the exact frequency of the trait. Numbers in the literature vary, however, a generally accepted rule of thumb is that approximately 50% of 50-year old men will have AGA, thus, affecting approximately 30 million men in the U.S.3,12,26.
Hereditary traits due to a single gene rarely occur with a frequency higher than 1:1000. If AGA were a dominant trait resulting from a single gene, then it would be predicted that only 270,000 individuals in the U.S. would be affected. As a general rule, inherited traits with a higher prevalence are due to more than one gene, therefore, the prevalence of AGA is a strong argument in favor of polygenic transmission28.
Second Argument: The Normal Gaussian Curve of Distribution
There is no generally accepted borderline between “normal” men and individuals with AGA. Any attempt to draw the line would be arbitrary, and in fact, all stages ranging from full hair growth to complete baldness are found in the population, with the transitions between these stages being fluid. The prevalence of AGA corresponds to a normal gaussian (bell) curve of distribution, typical of polygenic traits. If AGA were due to a single gene, there would be a clear-cut difference between the phenotypes, and we would expect a curve with two or more peaks28.
The distribution of AGA is best explained by a model of polygenic inheritance with a threshold effect. Individuals on the far left of such a curve represent those who carry only a few predisposing genes, and therefore enjoy a lifelong full head of hair. For those in the middle curve, there is a threshold for the manifestation of the trait, and the threshold is lowered by the presence of circulating androgens28. The essential role of androgens in the development of AGA is reflected in the nomenclature “andro” “genetic”, since it is believed that androgens alone cannot produce AGA without an inherited predisposition, and the inherited predisposition cannot produce AGA in the absence of androgens5,28.
Third Argument: Risk Increases with the Number of Family Members Already Affected
In 1946, Harris examined 900 men and found a “premature baldness” in 120 of them29. In this group, he determined the number of affected brothers in families with an affected father, as well as in families with an unaffected father. He showed that the number of affected brothers was higher in families that also had an affected father. If AGA were due to a single autosomal dominant gene, there should be no difference between the two groups. In contrast, a model of polygenic transmission would suggest that there should be a difference, since the risk of an individual developing AGA would increase with the number of family members already affected28.
Fourth Argument: Severely Affected Women Carry More Predisposing Genes that Mildly Affected Women
In 1964, Smith and Wells studied the first-degree relatives of 56 women with AGA30. Eighteen of these women had severe AGA, and interestingly, these 18 women had more affected first-degree relatives as compared to the entire group of 56 women. Smith and Wells postulated that the severely affected women were homozygous for a dominant gene, but they emphasized that this would not explain the fact that only 33% and not 100% of the fathers of these women were affected. This observation argues against the assumption of simple Mendelian inheritance, and also cannot be explained by the auxiliary hypotheses of diminished penetrance and variable expressivity. However, the model of polygenic inheritance provides an explanation for the results obtained by Smith and Wells: In the severely affected women, the number of predisposing genes is higher than in the mildly affected women, and therefore, more first-degree relatives will be affected28.
Fifth Argument: Affected Women Carry More Predisposing Genes Than Affected Men
In 1890, Jackson examined 1,200 individuals with “premature baldness”, consisting of 410 men and 790 women31. In the group of 410 men, he found a genetic influence from the father’s side in 236 cases, from both parents in 27 cases, and from the mother’s side in 9 cases. In the group of 790 women, he found 131 affected fathers and 240 affected mothers. This would be consistent with the assumption that for affected women, the maternal predisposition is more important than the paternal predisposition. However, the threshold concept provides an explanation that is more plausible than assuming homozygosity in severely affected women. Due to the higher androgen threshold present in women, an affected woman would carry a higher number of predisposing genes than a man affected to the same degree, and therefore, she would transmit a higher number of predisposing genes to her offspring28.
Summary
The discovery of genes directly implicated in the pathogenesis of inherited hair loss will have far-reaching implications for the treatment of affected individuals. Ultimately, it is hoped that discovery and modulation of the genes for inherited hair loss will provide novel therapeutic targets, and eventually eliminate these psychologically devastating disorders.
References
0. Ahmad, W., Ul Haque, M. F., Brancolini, V., Tsou, H. C., Ul Haque, S., Lam, H., Aita, V. M., Owen, J., deBlaquiere, M., Frank, J., Cserhalmi-Friedman, P. B., Leask, A., McGrath, J. A., Peacocke, M., Ahmad, M., Ott J., Christiano, A. M.: Alopecia Universalis Associated with a Mutation in the Human Hairless Gene. Science 1998; 279: 720-724.
1. Chernow, R.: Titan: The Life of John D. Rockefeller, Sr. Random House, New York 1998; 192: 407-408.
2. Sawaya, M.E., Hordinsky, M.K.: Advances in Alopecia Areata and Androgenetic Alopecia. Adv. Dermatol 1992; 211-226.
3. Schwartz, R.A., Janniger, C.K.: Alopecia Areata. Cutis 1997; 59: 238-241.
4. Kaufman, K.D.: Androgen Metabolism as it Affects Hair Growth in Androgenetic Alopecia. Dermatol. Clin. 1996; 14: 697-711.
5. Roberts, J.L.: Androgenetic Alopecia in Men and Women: An Overview of Cause and Treatment. Dermatol. Nurs. 1997; 9:379-386.
6. Drake, L.A., Dinehart, S.M., Farmer, E. R., Goltz, R.W., Graham, GAF., Hordinsky, M.K., Lewis, C.W., Pariser, D.M., Webster, S.B., Whitaker, D.C., Butler, B., Lowery, B.J., Price, V.H., Baden, H., DeVillex, R.L., Olsen, R., Shupack, J.L.: Guidelines of Care for Androgenetic Alopecia. American Academy of Dermatology. J. Am. Acad. Dermatol. 1996; 35: 465-469.
7. Sawaya, M.E., Price, V.H.: Different Levels of 5 Alpha-Reductase Type I and II, Aromatase and Androgen Receptor in Hair Follicles of Women and Men with Androgenetic Alopecia. J. Invest. Dermatol. 1997; 109: 296-300.
8. Ellis, J.A., Stebbing, M., Harrap, S.B.: Genetic Analysis of Male Pattern Baldness and the 5?-Reductase Genes. J. Invest. Dermatol. 1998; 110: 849-853.
9. Cash, T.F.: The Psychological Effects of Androgenetic Alopecia in Men. J. Am. Acad. Dermatol. 1992; 26: 926-931.
10. Maffei, C., Fossati, A., Rinaldi, F., Riva, E.: Personality Disorders and Psychopathologic Symptoms in Patients with Androgenetic Alopecia. Arch. Dermatol. 1994; 130: 868-872.
11. Spindler, J.R., Data, J.L.: Female Androgenetic Alopecia: a Review. Dermatol. Nurs. 4: 93-99, 1002.
12. Cash, T.F., Price, V.H., Savin, R.C.: Psychological Effects of Androgenetic Alopecia on Women: Comparisions with Balding Men and With Female Control Subjects. J. Am. Acad. Dermatol. 1993; 29: 568-575.
13. American Hair Loss Council, Chicago, IL.
14. Lander, E.S., Schork, N.J.: Genetic Dissection of Complex Traits. Science 1994; 265: 2037-2048.
15. Kruglyak, L., Lander, E.S.: High-Resolution Genetic Mapping of Complex Traits.
Am. J. Hum. Genet. 1995; 56:1212-1223.
16. Ott, J.: Complex Traits on the Map. Nature 1996; 379: 772-773.
17. Kruglyak, L., Lander, E.S.: Complete Multipoint Sib-Pair Analysis of Qualitative and Quantitative Traits. Am. J. Hum. Genet. 1995; 57: 439-454.
18. Weeks, D.E., Lathrop, G.M.: Polygenic Disease: Methods for Mapping Complex Diseases Traits. Trends Genet. 1995; 11: 513-519.
19. Knapp, M., Seuchter, S.A.,Baur, M.P.: Linkage Analysis in Nuclear Families. 2: Relationship Between Affected Sib-Pair Tests and Lod Score Analysis. Hum. Hered. 1994 4: 44-51.
20. Knapp, M., Seuchter, S.A., Baur, M.P.: Linkage Analysis in Nuclear Families. 1: Optimality Criteria for Affected Sib-Pair Tests. Hum. Hered. 1994; 44: 37-43.
21. Holmans, P., Craddock, N.: Efficient Strategies for Genome Scanning Using Maximum-Likelihood Affected-sib-pair Analysis. Am. J. Hum. Genet. 1997; 60: 657-666.
22. Kruglyak, L. Lander, E.S.: A Nonparametric Approach for Mapping Quantitative
Trait Loci. Genetics 1995; 139:1421-1428.
23. Fulker, D.W., Cherny, S.S.: An Improved Multipoint Sib-pair Analysis of Quantitative Traits. Behav. Genet. 1996; 25:527-532.
24. Risch, N.: Linkage Strategies for Genetically Complex Traits. II. The Power of Affected Relative Pairs. Am. J. Hum. Genet. 1990; 46:229-241.
25. Bergfeld, W.F.: Androgenetic Alopecia: An Autosomal Dominant Disorder. Am. J. Med. 1995; 98: 95S-98S.
26. Osborn, D.: Inheritance of Baldness. J. Hered.1916; 7: 347-355.
27. Küster, W, Happle, R.: The Inheritance of Common Baldness: Two B or not Two B? J. Am. Acad. Dermatol. 1984; 11:921-926.
28. Harris, H.: The Inheritance of Premature Baldness in Men. Ann. Eugenics 1946; 13: 172-181.
29. Smith, M.A. and Wells, R.S.: Male-Type Alopecia, Alopecia Areata, and Normal Hair in Women. Arch. Dermatol. 1964; 89: 155-158.
30. Jackson: Diseases of the Hair and Scalp. New York, 1890. (cited according to Galewsky, E. Erkrankungen der Haare und des Haarbodens, in Jadassohn J, editor: Handbuch der Haut und Geschlechtskrankheiten. 1932 Springer-Verlag, Vol. 13, pp.216-220.)
A History Lesson 1
In 1983, at a hair replacement symposium in Los Angeles, a single case report of transplanting only ten two-haired micrografts to refine an abrupt standard grafted hairline was presented. The speaker was careful to mention that harvesting and planting 10-15 micrografts in each standard grafting session, and placing them on the part-side only, would add only ten or fifteen minutes of additional time to the length of each surgical session.
No sooner had the presentation ended when a distinguished dermatologic surgeon rose from his seat and announced to the other three hundred members of the audience, “This lecture was the greatest waste of time I have ever been forced to sit and listen to. The hair transplant procedure is tedious and time-consuming enough without adding another fifteen minutes for only fifteen more hairs. Besides my patients are already very happy with their results. I hardly think a few more isolated single hairs would make any difference to them.” No one in the room rose to refute him.
One year later that presentation was published in the Journal for Dermatologic Surgery!2 Dr. Art Ulene, then the medical correspondent for the Today Show, read it, contacted the speaker, filmed the process, and broadcasted it to a national television audience. The rest, as they say, is history. Apparently those legions of supposedly “happy patients” concluded quite independently from their doctors, that those “few isolated single hairs” just might make them even happier that they already were! Unlike their surgeons, they saw a great deal of difference, indeed. Protestations of additional time and tedium meant nothing to them…that was the doctor’s problem. They cared only how they looked.
The future of hair replacement surgery will be determined, not by the doctor, but by the patient. The fully informed patient, the educated, savvy hair consumer is the most feared and powerful species in the free enterprise jungle of surgical hair replacement. His power comes from his knowledge. Comfortable and complacent surgeons should be wary. Though Dr. Art Ulene has retired, it will only be a matter of time before some other enterprising investigative, reporter decides once again to educate the “happy” hair consumer. Then, as in 1983, the rest will be history, and those who cannot remember it will be condemned to repeat it.
References
0. Marritt, E: Full text to be published in Hair Transplant Forum International.
1. Marritt, E: Single-hair Transplantation for Hairline Refinement: A Practical Solution. Journal for Dermatologic Surgery and Oncology Dec’84; 10(12): 962-66.
Acknowledgment: The authors would like to thank Rebecca Sipala, Nazia Rashid and Marie Rassman for their assistance in the preparation of this manuscript.