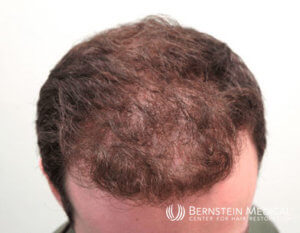
A 28-year-old male presents to the office with progressive hair loss for 3 years. His father and maternal grandfather are both bald. He has tried topical minoxidil 5% solution previously but could not comply with daily application. He has a history of OCD and panic disorder relating to needles. He has read on the internet that finasteride is the most effective FDA-approved therapy for male genetic thinning but is worried about the risk of sexual dysfunction and other side effects. On examination with trichoscopy the patient has miniaturized hairs at the frontal and top scalp. A hair pull test is negative.
Q: What would be the next best step?
- Biopsy of the scalp
- Laboratory bloodwork
- Start finasteride 1mg oral daily
- Retrial of topical Rogaine
- Platelet-Rich Plasma
- Oral minoxidil
Answer F. The presentation and examination of this patient indicate genetic thinning and so a biopsy or laboratory bloodwork are unnecessary. Given the history of psychiatric disorder in this patient and the strong fear regarding side effects of finasteride, this patient should not be encouraged to begin this medication. If the patient later decides he wants finasteride, a second opinion from a primary doctor or psychiatrist would be highly advisable. The patient had already shown poor compliance with topicals and has needle-phobia and so neither Rogaine nor PRP would be appropriate. Instead, you should consider a discussion about oral minoxidil, complete with information regarding its off-label use and side-effect profile.
Q: What other patients are good candidates for off-label oral minoxidil?
- Patients who wash their hair infrequently and find that daily application of topical minoxidil leaves a residue in their hair or that it is just too inconvenient to use.
- Patients who are having side effects of itching or flaking scalp with topical minoxidil.
- When other treatments do not seem to be working well enough.
- Patients who are having side effects to finasteride or just choose not to use this medication.
- Patients who want to maximize their treatment response.
Answer All the above. All of these are suitable reasons to consider a discussion of off-label low-dose oral minoxidil once appropriate informed consent is obtained.
Q: The patient is amenable to starting oral minoxidil. What dose would you initially prescribe?
- 1.25mg oral minoxidil daily
- 2.5mg oral minoxidil daily
- 5mg oral minoxidil daily
- 10mg oral minoxidil daily
Answer B. While studies show that doses of 2.5, 5 or 10mg are effective doses for hair loss in men, initially new medications are often started at the lowest effective dose and then slowly titrated upward after the patient has shown good ability to tolerate the new medication. In female patients, 0.625mg or 1.25mg are the recommended starting doses.
Q: The patient’s girlfriend uses topical minoxidil and asks if she can use oral minoxidil too. You respond:
- Yes, but you will need to stop the topical minoxidil first to avoid overdose
- Yes, but you should initially consider using both topical and oral together
- Yes, and you should overlap use of the topical with the oral while it begins to take effect and to see if you can tolerate it before stopping the topical
- No, it would be better if you continue with the topical version if you are tolerating it well since oral minoxidil should be avoided in pre-menopausal women
Answer B, C. Since oral minoxidil is an off-label treatment, formal guidelines do not yet exist on how to manage patients currently on the topical form and wishing to add-in or switch to the oral version. Most physicians will advise overlap for 3-6 months to make sure there are no side effects before the prior topical medication is discontinued. Since oral minoxidil was historically prescribed at 20-40mg doses for blood pressure, it is unlikely that a low-dose oral minoxidil along with the topical would cause overdose. While the side effect profile is not fully understood for low-dose oral minoxidil, it can be added to a woman’s hair loss regimen if appropriate informed consent about potential risks is reviewed. Neither oral nor topical minoxidil should be used in pregnant women.
Q: The girlfriend wants to begin oral minoxidil. You begin the medication at a 0.625mg oral daily dose with a plan to increase to 1.25mg daily if well-tolerated. What medication would you consider adding in conjunction with oral minoxidil?
- Spironolactone 25mg PO daily
- Oral finasteride 1mg PO daily
- Birth control if the patient is having unprotected sex
Answer A and C. Oral minoxidil should not be used in pregnant women. If a woman plans to become pregnant while on oral minoxidil, then she should consider stopping the medication 3 months prior to conceiving. If a woman is having unprotected sex, it would be advisable to also begin some form of contraception. Many women also report water retention and weight gain while on low-dose oral minoxidil. Spironolactone at a 25mg oral daily dose may act as a weak diuretic to help mitigate this side effect.
Q: Both your male patient and his girlfriend are eager to try oral minoxidil as an off-label therapy. What other conditions should you ask about specifically regarding this medication that may warrant caution before prescribing?
- Are you taking any heart or blood pressure medication?
- History of EKG changes or arrhythmia?
- Hypotension?
- Hypertension?
- Renal problems?
Answer All the above. Patients with a cardiac history or renal disease should be warned that oral minoxidil has been associated with reflex tachycardia, EKG changes, pericardial effusion, and congestive heart failure. Sodium and water retention are side effects that lead to weight gain or leg/ankle swelling and very infrequently can cause pulmonary edema, especially in patients with renal conditions. These cardiac and renal side effects are mostly seen at 10 to 40mg daily dosages and the risk is greatly diminished at the low-dose version used for hair loss. Nonetheless, caution should be advised in these higher risk patients. Ask about hypotension, as these patients may be more sensitive to the side effects of oral minoxidil. Ask about hypertension as well, as hypertensive patients are likely to be on other medications that may interact with minoxidil (or might need appropriate medication if the high blood pressure has not yet been treated).
The most common side effect reported is hypertrichosis which is often described as mild and manageable. Other less frequent side effects include dizziness/postural hypotension and blood pressure changes. Although significant cardiopulmonary events are very unlikely in a healthy person at the low dosages of oral minoxidil recommended, providers should remain vigilant and may want to refer patients on oral minoxidil for regular blood pressure, heart rate, and fluid retention monitoring with their primary care provider. At a minimum, the patient’s PCP should be aware that the patient is taking this systemic medication.
Q: Oral minoxidil is contra-indicated in which of the following condition(s)? Circle all that apply:
- Parkinson’s disease
- Chronic Lymphocytic Leukemia
- Pheochromocytoma
- Sickle Cell Trait
- Ulcerative Colitis
Answer C. Minoxidil tablets are contraindicated in pheochromocytoma because it may stimulate secretion of catecholamines from the tumor through its antihypertensive action.
If you are experiencing gradual hair thinning, please contact our office for an appointment. Call 212-826-2400 or schedule a consult here.





