Robert M. Bernstein, M.D.
Surgery of the Skin: Procedural Dermatology, Chapter 30. Mosby Elsevier Inc., London UK. 2015: 503-533.
Summary Box
- Follicular unit hair transplantation (FUHT) is a method of hair restoration surgery that relocates permanent donor hair to balding areas in naturally occurring follicular unit hair groupings. The small size of the units allows for tremendous versatility in their placement, the creation of hair patterns that closely mimic nature, and the transplantation of a large enough number of grafts so that a full restoration can often be achieved in just one or two sessions.
- In follicular unit transplantation (FUT), the donor hair is harvested from the back and sides of the scalp via a long, thin strip and then dissected into individual follicular units using stereo-microscopes. In follicular unit extraction (FUE), individual follicular units are removed directly from the donor area through tiny round incisions. Follicular unit hair transplantation requires large numbers of grafts, so it is important to accurately assess patients’ donor reserves in the initial planning. Densitometry enables the physician to estimate the total amount of movable hair, the size of the individual follicular units, and the degree of miniaturization in both the donor and recipient areas; scalp laxity assessment is also crucial to the surgical planning.
- Great care should be taken to screen for patients who are too young for hair restoration surgery or whose hair loss may be diffuse, making the donor supply inadequate for a hair transplant.
- Identifying androgenetic alopecia often proves difficult in women as other medical conditions can have a similar presentation. Options for medical treatment are more limited in women and many experience diffuse hair loss, a relative contraindication to surgery.
- Determining the right size and location of the donor area maximizes the yield and prevents problems such as widened and/or visible scars.
- The follicular units obtained from the donor area are delicate structures. Careful dissection, gentle handling, and adequate hydration are essential to their survival. In order to dissect and place the thousands of grafts often required in follicular unit transplantation, the surgical team must be proficient in stereomicroscopic dissection and insertion techniques – skills that can take a year or more to develop. Follicular unit extraction requires its own sets of skills, some of them now being performed robotically.
- Follicular unit transplantation requires an astute aesthetic sense for optimal hairline design, correct angling and distribution of grafts, and the establishment of a master plan that anticipates further hair loss and is consistent with the long-term goals of the patient.
INTRODUCTION
Within the past two decades, follicular unit hair transplantation has transformed hair restoration surgery from a cosmetically unpredictable procedure to one that can produce consistently natural results. The key to its effectiveness lies in the fact that scalp hair tends to grow in tiny bundles, called “follicular units,” rather than individually. ((Bernstein RM, Rassman WR, Szaniawski W, et al. Follicular transplantation. Int J Aesthetic Rest Surg 1995;3:119–32.)) By working with these naturally occurring units, instead of larger or smaller grafts, one can create as natural a look as possible, while minimizing the transplant wound size and accelerating postoperative healing.
Follicular units are made up of 1–4 terminal hairs, 1–2 vellus hairs, their associated sebaceous glands, neurovascular plexus, an erector pilorum muscle, and a circumferential band of adventitial collagen, the perifolliculum. ((Headington JT. Transverse microscopic anatomy of the human scalp. Arch Dermatol 1984;120:449–56.)) The tendency of scalp hair to grow in this way, rather than as single hairs, can be most easily demonstrated by clipping the hair to approximately 1 mm in length and then viewing it with a densitometer (Fig. 30.1) at ×30 magnification in a 10 mm field. ((Rassman WR, Pomerantz MA. The art and science of minigrafting. Int J Aesthetic Rest Surg 1993;1:27–36.)) What this also reveals is that these compact units are surrounded by significant amounts of non-hair-bearing scalp (Fig. 30.2). ((Limmer B. Thoughts on the extensive micrografting technique in hair transplantation. Hair Transplant Forum Int 1996;6:16–18.)) Including this extra skin in the dissection – as do transplants with larger grafts, such as plugs and minigrafts – requires a larger recipient wound, as well as risking visible scarring in the skin around the grafts and distortions of the growing hair.
Follicular unit hair transplantation takes advantage of the anatomic proximity of the hairs within each unit to:
- Keep the recipient site wound size to a minimum
- Prevent skin surface change in the recipient area
- Facilitate postoperative healing
- Enable grafts to be placed very close together
- Permit large numbers of grafts to be safely transplanted in a single session (because of the small recipient wound size).1, ((Bernstein RM, Rassman WR. The logic of follicular unit transplantation. Dermatol Clin 1999;17:277–95.))
The ability to insert up to four hairs in a tiny recipient site is especially valuable cosmetically and has been instrumental in eliminating the see-through quality of older micrografting techniques where follicular units were not kept whole. When using follicular units, the transplanted hair will look natural – given that the transplant surgeon makes the right aesthetic decisions about graft placement, angling, and distribution. ((Bernstein RM, Rassman WR. Follicular transplantation: patient evaluation and surgical planning. Dermatol Surg 1997;23:771–84.)), ((Bernstein RM, Rassman WR. The aesthetics of follicular transplantation. Dermatol Surg 1997;23:785–99.))

 FIGURE 30.1 (A) Hair densitometer (B) Video-densitometer
FIGURE 30.1 (A) Hair densitometer (B) Video-densitometer FIGURE 30.2 Natural hair groupings as seen through a densitometer (magnification x30).
FIGURE 30.2 Natural hair groupings as seen through a densitometer (magnification x30).Follicular unit hair transplantation (FUHT) is the general term used for hair restoration surgery using individual follicular units. In follicular unit transplantation (FUT), the donor hair is harvested from the back and sides of the scalp via a long, thin strip and then dissected into individual follicular units using stereomicroscopes. ((Limmer BL. Elliptical donor stereoscopically assisted micrografting as an approach to further refinement in hair transplantation. Dermatol Surg 1994;20:789–93.)), ((Seager D. Binocular stereoscopic dissecting microscopes: should we use them? Hair Transplant Forum Int 1996;6:2–5.)) In follicular unit extraction (FUE), individual follicular units are removed directly from the donor area through tiny round incisions. ((Rassman WR, Bernstein RM, McClellan R, et al. Follicular unit extraction: minimally invasive surgery for hair transplantation. Dermatol Surg 2002;28:720–7.))
The purpose of this chapter is to describe the basic technical skills and aesthetic judgments involved in FUHT. It should be taken as a framework for physicians interested in learning the procedure, not an exhaustive review of the various surgical techniques used in FUHT or of other hair restoration methods. The chapter will not cover mini-micrografting; ((Stough DB, Haber RS, editors. Hair replacement: surgical and medical. St Louis: Mosby; 1996.)) the combining of large and small grafts; ((Unger WP. Different grafts for different purposes. Am J Cosmet Surg 1997;14:177–83.)) laser hair transplantation; ((Unger WP. Laser hair transplanting 1997. Am J Cosmet Surg 1997;14:143–8.)), ((Bernstein RM, Rassman WR. Laser hair transplantation: is it really state of the art? Lasers Surg Med 1996;19:233–5.)) scalp reductions; ((Norwood OT. Scalp reductions: are they necessary? Hair Transplant Forum Int 1993;3:1–7.)), ((Bernstein RM. Are scalp reductions still indicated? Hair Transplant Forum Int 1996;6:12–13.)) scalp lifts; ((Swinehart JM, Brandy DA. Scalp lifting: anatomic and technical considerations. J Dermatol Surg Oncol 1994;20:600–12.)) or flaps. ((Epstein JS, Kabaker SS. Scalp flaps in the treatment of baldness: long-term results. Dermatol Surg 1996;22:45–50.)) These procedures are infrequently used today and have been detailed in many excellent publications, as well as in two comprehensive textbooks.11, ((Unger WP, Shapiro R. Hair transplantation. 4th ed. New York: Marcel Dekker; 2004.))
When learning FUHT, the importance of hands-on experience and beginning with small sessions until skills develop cannot be overemphasized. Having a well-trained team of assistants is also important to a successful outcome, particularly for those procedures involving a large number of grafts.
Although simple in concept, FUHT has many nuances and complexities. Those wishing to perform FUT in their clinical practice are encouraged to join the International Society of Hair Restoration Surgery (ISHRS) and attend its annual meeting, to subscribe to Hair Transplant Forum International (the trade publication for hair restoration surgeons), and to follow relevant medical literature. Although it is an independent board, certification by the American Board of Hair Restoration Surgery (ABHRS) indicates a basic competency in the field. Certification requires several years of clinical experience and passing both oral and written examinations. The process of preparing for ABHRS certification is a worthwhile endeavor and recommended for those serious about surgical hair restoration.
The term follicular unit hair transplantation (FUHT) should now be used to encompass all hair restoration procedures that utilize naturally occurring individual follicular units exclusively in the surgery, regardless of how these units are harvested. Follicular unit transplantation (FUT) refers to the technique of obtaining follicular units through the microscopic dissection of a donor strip and follicular unit extraction (FUE) refers to harvesting individual units directly from the scalp. In 2011, the first follicular unit extraction procedure was performed using a video-guided robotic device and a new acronym was introduced into the field of hair transplant surgery, R-FUE.
HISTORICAL VIGNETTE
Reports of successful hair transplants appeared as early as 1930 in the Japanese literature, beginning with Sasagawa’s hair-shaft insertion procedure ((Sasagawa M. Hair transplantation. Jpn J Dermatol 1930;30:473, [in Japanese].)) and then Okuda’s technique of using 2–4 mm punches for the treatment of various alopecias of the scalp, eyebrows, and moustache. Okuda made the important observation that using smaller punches in the recipient area improved cosmetic results. ((Okuda S. Clinical and experimental studies of transplantation of living hairs. Jpn J Dermatol Urol 1939;46:135–8, [in Japanese].))
By 1943, Tamura had treated 137 cases of non-androgenetic alopecia of various etiologies using techniques very similar to modern day hair transplantation. ((Tamura H. Pubic hair transplantation. Jpn J Dermatol 1943;53:76, [in Japanese].)) For instance, he harvested donor grafts by making an elliptical incision that was sutured closed, prepared recipient sites with a thick needle, stored grafts in physiologic saline, and observed postoperative telogen effluvium (shedding). Most significantly, Tamura demonstrated that single-hair grafting resulted in growth practically indistinguishable from naturally grown hair – and much more natural looking than transplants using larger grafts. Unfortunately, it took several decades before Western surgeons would apply Tamura’s insights to their hair restoration procedures.
The first hair transplant in the USA was performed by Dr Norman Orentreich in 1952, with grafts measuring 6–8 mm in diameter, ((Orentreich N. Autographs in alopecias and other selected dermatologic conditions. Ann NY Acad Sci 1959;83:463–79.)) significantly larger than those of either Tamura or Okuda. At first, incredulous editors rejected Orentreich’s work, not believing that hair transplantation was even possible. In 1959, the Annals of the New York Academy of Science finally agreed to publish his work. The paper laid out the concept of “donor dominance” – the idea that grafts continue to show the characteristics of the donor site after they have been transplanted to a new location. This remains the basic tenet of all hair transplantation surgery. Yet, while donor dominance insured that transplanted hair could grow, it did not guarantee that the results would look natural.
Not until 40 years later would hair transplants in the USA start to produce consistently natural-looking results and promise predictable cosmetic improvements in most patients. It was a slow evolution, but the large grafts used throughout the 1960s and 1970s eventually gave way to mini-grafts in the 1980s3 and mini-micrografting in the early 1990s. ((Rassman WR, Carson S. Micrografting in extensive quantities; the ideal hair restoration procedure. Dermatol Surg 1995;21:306–11.)) The stage was then set for follicular unit transplantation. First appearing in the medical literature in 1995,1 it quickly emerged as the gold standard – supplanting mini-micrografting in the treatment of androgenetic alopecia and rendering other well-established procedures such as scalp reductions, scalp lifts, and flaps virtually obsolete.
So swift was the ascent of FUT that the two standard textbooks on surgical hair restoration, published in 1995, ((Unger WP. Hair transplantation. 3rd ed. New York: Marcel Dekker; 1995.)) and 1996,11 as well as the most comprehensive text on trichology, published in 1997, ((Camacho F, Montagna W. Trichology: diseases of the pilosebaceous follicle. Madrid: Aula Medica; 1997.)) make not one mention of the terms “follicular unit” or “follicular unit transplantation.” At the 1996 meeting of the ISHRS, three 7-min presentations on the procedure were given; but at the 2002 meeting, FUT was the subject of entire seminars and workshops and suffused every aspect of the week-long gathering.
The follicular unit was first defined by Headington in his landmark 1984 paper entitled “Transverse microscopic anatomy of the human scalp.”2 Follicular unit transplantation had its origins in the microscopic dissection techniques of Dr Limmer in 1988 that was described in his paper, “Elliptical donor stereoscopically assisted micrografting as an approach to further refinement in hair transplantation” in 1994.8 The term “follicular unit” was introduced into the hair transplant literature by Bernstein and colleagues in 1995. The conceptual framework for FUT was mapped out by these authors in their 1995 publication “Follicular transplantation”1 and in the paired articles “Follicular transplantation: patient evaluation and surgical planning” and “The aesthetics of follicular transplantation” in 1997.6,7
The name “follicular unit transplantation” was formalized by a group of hair restoration surgeons in 1998. ((Bernstein RM, Rassman WR, Seager D, et al. Standardizing the classification and description of follicular unit transplantation and mini-micrografting techniques. Dermatol Surg 1998;24:957–63.)) In this paper, the procedure was precisely defined and included the two basic techniques: single-strip harvesting and stereomicroscopic dissection, as integral parts of the procedure. However, because follicular units can now be harvested directly from the donor area without the necessity of a strip incision (using follicular unit extraction10), the original definition has become too restrictive.
PREOPERATIVE PREPARATION
Patient evaluation and surgical planning
Diagnosis and classification of androgenetic alopecia
The diagnosis of androgenetic alopecia in men is usually straightforward. It is made by observing a “patterned” distribution of hair loss. It is confirmed by the presence of miniaturized hairs in the areas of thinning. The diagnosis is supported by a history of baldness in the family. ((Kuster W, Happle R. The inheritance of common baldness: two B or not two B? J Am Acad Dermatol 1984;11:921–6.)) In women, the diagnosis is more complex since the most common presentation, a diffuse pattern, can be mimicked by a host of non-androgenetic etiologies.
 FIGURE 30.3 Historic view of follicular units corresponding to the natural hair groups observed on the scalp surface. Transverse section taken at the level of sebaceous glands (H&E; original magnification x40).
FIGURE 30.3 Historic view of follicular units corresponding to the natural hair groups observed on the scalp surface. Transverse section taken at the level of sebaceous glands (H&E; original magnification x40).(From Headington JT. Transverse microscopic anatomy of the human scalp. Arch Dermatol 1984; 120:449–456 and Bernstein RM, Rassman WR. The logic of follicular unit transplantation. Dermatol Clin 1999; 17:277–295.)
Miniaturization – the progressive diminution of the hair shaft’s diameter and length in response to androgens – can be most readily observed with a densitometer,11 a handheld instrument that magnifies a small area of the scalp where the hair has been clipped to about 1 mm. One type, the Rassman densitometer,3 magnifies by ×30 in a visual field of 10 mm2, making it easy to identify miniaturization and calculate hair density (Figs 30.1–30.3). With video-densitometry (Fig. 30.1B), the doctor can project the image onto a computer screen so that it is immediately visible to the patient. It can also provide a permanent digital record of the person’s density and miniaturization status. ((Bernstein RM, Rassman WR. Densitometry and video-microscopy. Hair Transplant Forum Int 2007;17:1, 49–51.))
When describing hair loss, particularly in the early phases, it is preferable to think in terms of changes in volume rather than density. Density is simply the number of hairs per unit area, whereas volume reflects both hair shaft diameter as well as the absolute numbers of hairs. ((Bernstein RM. Measurements in hair restoration. Hair Transplant Forum Int 1998;8:27.))
A new device, called the Cross Section Trichometer, takes both of these factors into account by measuring the cross-sectional area of a demarcated bundle of hair. ((Cohen BH. The cross-section Trichometer: a new device for measuring hair quantity, hair loss and hair growth. Dermatol Surg 2008;34(7):900–11.)) This instrument measures hair mass – the cross-sectional area of a bundle of hair present in a premeasured area of scalp. It detects small changes in both hair density and diameter. Since the earliest sign of hair loss results in volume changes caused by individual hairs decreasing in size (miniaturization) without a decrease in number, devices that can assess hair shaft diameter are useful for identifying early hair loss. Only in more advanced balding will the actual number of hairs start to decrease.1
 FIGURE 30.4 Norwood classification.
FIGURE 30.4 Norwood classification.(From Norwood OT. Male pattern baldness: classification and incidence. South Med J 1975; 68:1359–1365.)
The Norwood classification of male pattern hair loss, published in 1975, remains the most widely used. ((Norwood OT. Male pattern baldness: classification and incidence. South Med J 1975;68:1359–65.)) It defines two major patterns and several less common types (Fig. 30.4). In the regular Norwood pattern, two areas of hair loss – a bitemporal recession and thinning crown – gradually enlarge and coalesce until the entire front, top, and crown (vertex) of the scalp are bald. A less common Norwood variety, Norwood class A, is characterized by a distinctly anterior-to-posterior progression, usually resulting in baldness on the front and top of the scalp, but more limited loss in the crown. In both patterns, the sides and back tend to resist androgenetic changes, though the sides may exhibit significant thinning in senile alopecia.
Two other types of genetic hair loss not emphasized in the literature – diffuse patterned alopecia (DPA) and diffuse unpatterned alopecia (DUPA) – pose the greatest challenge both for diagnosis and patient management. A thorough familiarity with all of these patterns is essential for diagnosing androgenetic alopecia and planning FUT (Table 30.1).
| Table 30.1 Classification of androgenetic alopecia | |
| Norwood classes | Regular classes I–VII Type A variant (IIA–VA) Variations: persistent frontal forelock; ((Marritt E, Dzubow L. The isolated frontal forelock. Dermatol Surg 1995;21:523–38.)) persistent frontal hairline |
| Diffuse androgenic alopecias | Diffuse patterned alopecia (DPA) Diffuse unpatterned alopecia (DUPA) |
| Senile alopecia | |
| Female pattern alopecia | Ludwig classes I–III |
DPA is an androgenetic alopecia that manifests as diffuse thinning in the front, top, and vertex, with a stable permanent zone. In DPA, the entire top of the scalp gradually miniaturizes without passing through the typical Norwood stages. DUPA is also androgenetic but lacks a stable permanent zone. It affects men much less often than DPA.
DUPA tends to progress more rapidly than DPA and eventuates in a horseshoe pattern resembling the Norwood class VII. However, unlike the Norwood class VII, the DUPA horseshoe can look almost transparent due to the low density of the back and sides. Differentiating between DPA and DUPA is very important because DPA patients often make good transplant candidates, whereas DUPA patients almost never do, as they inevitably have extensive hair loss without a stable zone for harvesting.6
The early warning signs of DUPA include:
- A rapid decrease in hair volume and a change in hair texture at an early age, often in the teens
- The maintenance of an adolescent hair pattern and persistent frontal hairline in spite of dramatic volume change
- A see-through donor area (most easily visualized when the hair is wet or lifted up)
- Significant miniaturization of the donor area (>35%)
- A donor density of <1.5 hairs per mm2.
The sensitivity of densitometry in detecting early DUPA justifies its routine use in the evaluation of hair loss. Once a diagnosis of DUPA is considered, any decisions regarding surgical hair restoration should be postponed. The possibility of missing a DUPA diagnosis is one of the most powerful arguments against performing hair transplantation at an early age.
Both DPA and DUPA occur in women but, in contrast to men, DUPA is far more common. As with men, women with DUPA do not make good transplant candidates (except possibly when donor hair is used solely to soften the frontal edge of a wig). Indeed, the higher DUPA incidence in women explains why a smaller proportion of women than men qualify as transplant candidates. Interestingly, the most common classification used for women, the Ludwig classification, does not differentiate between DPA and DUPA. ((Ludwig E. Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br J Dermatol 1977;97:247–54.))
There is a home test that screens for variations in the androgen receptor gene on the X chromosome, a gene associated with male pattern hair loss. Its purpose is to identify persons at increased risk of developing hair loss before it is clinically apparent so that medical intervention can be started early, when it is most effective. The presence of one of the variants indicates a moderately increased risk for the development of androgenetic alopecia and the other indicates a substantially decreased risk of becoming bald. A similar screening test is also available for the detection of hair loss in women. ((Keene S. A genetic test for androgenetic alopecia. Hair Transplant Forum Int 2008;18:91–3.))
It is important to realize that these markers reveal an association with hair loss and that a cause and effect relationship has not been proven. A danger is that patients may overreact to this relatively incomplete information. It is best to have the tests performed under a doctor’s supervision so that the results can be put in the context of other information that the physician gleans through a careful history and physical and so that the patient may be properly counselled.
| Box 30.1 Non-androgenetic causes of diffuse hair loss in women |
|
It is important to emphasize that, because a wide variety of medical conditions can produce diffuse hair loss, a nonandrogenetic differential must be considered in all cases of unpatterned alopecia. This is particularly relevant in evaluating women since unpatterned hair loss is the rule rather than the exception (Box 30.1). The following laboratory tests are often useful when a non-androgenetic cause for diffuse hair loss is suspected: chemistry screen, complete blood count, serum iron, total iron-binding capacity, triiodothyronine, thyroxine, thyroid-stimulating hormone, antinuclear antibody, and serologic test for syphilis.
In women, diffuse or patterned hair loss may be a sign of excessive androgen production. A further medical evaluation is indicated when the hair loss is associated with any of the following: cystic acne, irregular menses, hirsutism, virilization, infertility, or galactorrhea. Serum levels of dehydroepiandrosterone (DHEA) sulfate, androstenedione, total and free testosterone, and prolactin can serve as a useful screen. ((Olsen EA. Female pattern hair loss. J Am Acad Dermatol 2001;45: S70–80.))
When the diagnosis of androgenetic alopecia is uncertain, further diagnostic information can be gleaned from a hairpull test for telogen effluvium, a potassium hydroxide mount and culture for fungus, a microscopic examination of the hair bulb and shaft, and a scalp biopsy (sectioned horizontally). ((Chartier MB, Hoss DM, Grant-Kels JM. Approach to the adult female patient with diffuse non-scarring alopecia. J Am Acad Dermatol 2002;47:809–18.)) A dermatologic consultation is warranted whenever the diagnosis of hair loss is unclear.
Who is a candidate for hair transplantation?
| Box 30.2 Surgical patient selection for androgenetic alopecia |
|
| a Many non-androgenetic causes of hair loss can be treated with hair transplantation b Some are relative contraindications. |
In all cosmetic procedures, a successful outcome depends on proper patient selection (Box 30.2). In surgical hair restoration, proper timing is also crucial. There are no medical or surgical advantages to transplanting at an early age. The popular rationale that transplants should be performed at a young age so that “no one will notice” does not make sense: if there is nothing to notice from the transplant, why perform it in the first place? FUHTs heal so quickly and the hair growth is so gradual that, once the short postoperative period ends, the process is almost always undetectable. When hair loss is early, medical therapy is the treatment of choice.
Patients seeking hair transplantation while still in their early 20s invariably wish to have their adolescent hairline and original density restored. Since neither of these goals can be achieved surgically without compromising the patient’s future appearance, the procedure should not be performed at this age. It should be explained that hair transplantation moves, rather than creates, hair. It does not increase overall hair volume. In fact, the challenge is to have the pattern of the transplanted hair take into account the person’s ever-decreasing total hair mass. In other words, patients who will have significant balding need to have built-in temporal recession, and possibly a crown that is significantly thinner than the surrounding hair, for the transplant to look natural over time. Unfortunately, neither of these is generally acceptable to a young man in his early 20s.
Another reason to postpone transplants for those under 25 years, or with only limited hair loss, is to give medications – minoxidil and finasteride – adequate time to work. As patients may continue to show regrowth for 1–2 years after treatment is initiated, they should in general use medical therapy at least this long before surgery is considered. It is inappropriate, therefore, to start younger patients on minoxidil and finasteride and schedule surgery at the same time. Fortunately, minoxidil and finasteride work best in younger patients, and especially those with large areas of miniaturized hair, rather than areas that are totally bald. On the other hand, for older patients who would be taking medication to maintain rather than regrow hair, and for those whose advanced state of hair loss makes results from minoxidil and finasteride unlikely, the medications may be prescribed concomitantly with surgery.
With the availability of finasteride, medication plays an increasingly important role in the management of androgenetic hair loss. Although the incidence is small (2–4%), the fear of possible sexual side-effects has turned many against this drug. Some also consider surgery a more conservative form of treatment than the potential lifetime use of medication. Because of these concerns and the pressure from patients for surgery’s apparent “quick fix,” the physician should take time to thoroughly discuss the pros and cons of medical therapy.
In the Norwood classification, class I represents a normal adolescent pattern and class II a normal non-balding adult. Therefore, as a minimum, patients should qualify as class III before transplants are contemplated. Early class III patients will often benefit from medication alone, so this should be considered first.
Although the opportunities for a complete restoration in a person who is extensively bald (i.e., a Norwood class VII) is limited, extensive balding is not necessarily a contraindication to surgery. If the person’s donor zone is stable (miniaturization of <20%) and the patient’s expectations are commensurate with his or her limited available donor supply, a hair transplant may be considered. As long as the patient is in good health, there is no upper age limit for surgery. In fact, older patients tend to have the most reasonable expectations and are often the most satisfied. Even a small amount of hair can make a dramatic improvement in the appearance of a person who has been bald for many years.
There are few absolute medical contraindications to surgical hair restoration, particularly because it is an outpatient procedure not requiring general anesthesia. Relative contraindications include: bleeding disorders, drugs that increase bleeding such as clopidogrel (Plavix), immunodeficiency, unstable arrhythmias, chronic obstructive pulmonary disease, sensitivity to local anesthetics or epinephrine, a history of keloid formation or connective tissue disease, and major psychiatric disorders. When in doubt about the patient’s medical condition, it is always best to get medical clearance from their primary physician.
Patients should be able to tolerate being in the surgical chair for a good part of the day; consequently, back and neck problems as well as claustrophobia can sometimes make the procedure problematic. Special care should be taken when evaluating patients with significant psychiatric issues, particularly depression, trichotillomania, body dysmorphic disorder, or severe obsessive compulsive disorder. In such cases, a psychiatrist or psychologist should participate in the decision-making process.
Possibly the most difficult aspect of the consultation is managing patients’ expectations. It is almost a cliché to say that they must have “reasonable expectations,” but ensuring this is the essence of the evaluation. The surgeon cannot stress strongly enough that because hair restoration procedures move rather than create hair, the resulting transplanted density will be significantly less than the person’s non-balding density. It is also important to emphasize that it is the patient’s own hair from the sides and back of the head that will be transplanted. Therefore, the final result can be best described with reference to the texture, color, curl, and other qualities of the person’s existing hair. It is also helpful to provide prospective patients with printed material and photos, and to make past patients available for them to speak with or meet.
Assessing the patient’s donor supply
The main factors in determining total donor reserves are donor density, scalp laxity, and the physical size of the donor area.
Donor density
The number of follicular units in the mid-portion of the donor area of a normal Caucasian man who has not had surgery is approximately 70–95 follicular units per cm2.7 In the first procedure, therefore, the donor area should yield approximately 70–95 follicular grafts per cm2 of scalp, provided there is no significant wastage during surgery. The average yield declines when longer strips are taken because density decreases towards the sides. In some people, density varies dramatically throughout the donor area and this must be accounted for in the surgical planning. Follicular unit density also varies according to race; therefore the number of grafts obtained per unit area of donor tissue will exhibit racial variability and must be accounted for in the surgical planning.6
In general, as hair density increases (hairs/cm2) the number of hairs per follicular unit increases (hairs/FU) rather than follicular units being spaced closer together (FU/cm2).7 If the scalp has been stretched from previous transplants, scalp reductions, or scalp lifts, the follicular units will be spaced further apart.
Donor scarring from previous surgeries will also significantly diminish the donor yield. All donor harvests, no matter how perfectly executed, entail some loss of hair. In addition, the angle of the hair surrounding the scar will be altered slightly, creating more transection in any subsequent harvest.
A person can lose a substantial amount of hair volume – either due to actual loss of hair or to miniaturization – before it becomes noticeable. When hair is blond or white, it takes longer for thinning to show. Thinning is most evident in persons with black hair and white skin. For those with average density and average hair attributes, approximately half of the hair in the donor area may be moved without a significant change in appearance.
Scalp laxity
Scalp laxity (looseness) is an important factor in determining a person’s total available donor hair. There appears to be two main factors that contribute to laxity: redundancy and distensibility. Redundancy is simply extra scalp skin. Distensibility is the ability of the scalp to stretch. In those with lax scalps due to redundancy, harvesting the donor strip merely removes some of the extra scalp tissue while only slightly affecting density (hairs/mm2); therefore, the yield will be high, as the density will not decrease significantly over subsequent procedures. With little redundancy, however, each procedure stretches the skin, measurably decreasing density. The limitations of a tight scalp are usually not apparent in the initial surgery, but in subsequent procedures, it can become much harder to perform a nontension closure or, in the face of decreased density, to harvest a significant amount of hair. Therefore, the long-term goals of those with tight scalps must reflect a more limited donor supply.
Patients with very loose scalps, due to increased distensibility, tend to heal with widened scars and so often make poor candidates for procedures using a linear incision. Some, in fact, may have undiagnosed connective tissue disease. ((Bernstein RM, Rassman WR. The scalp laxity paradox. Hair Transplant Forum Int 2002;12:9–10.)) Follicular unit extraction should be considered in cases of both very loose and very tight scalps; however, it is important to keep in mind that although FUE is often easy in patients with average to tight scalps, it is sometimes very difficult in patients with loose scalps, as the skin is difficult to stabilize and the follicular units may shred during the extraction process.10
Donor dimensions
The mid-portion of the harvestable donor zone generally lies over the occipital protuberance and extends laterally to within 3 cm of the temple hairline on either side. This distance is approximately 32–35 cm. Besides limiting the length of the incision, temple recession can signal extensive balding and portends a diminished donor supply. The height of the permanent zone can vary markedly from person to person and should be carefully measured. Hair should be harvested only where it is stable, i.e., where it lacks significant miniaturization. An often overlooked sign is the “ascending” hairline, characterized by miniaturization in the lower margin of the permanent zone at the back of the scalp. Both receding temples and an ascending posterior hairline indicate a shrinking donor zone and mandate conservative surgical planning.
Planning the first hair transplant session
Ideally, the main goals of the first transplant session should be:
- To establish the frontal hairline and frame the face
- To provide coverage to all bald areas of the scalp, from the frontal hairline to the vertex transition point ((Beehner M. Nomenclature proposal for the zones and landmarks of the balding scalp. Dermatol Surg 2001;27:375–80.))
- To create sufficient density so that the results will look natural after one session, though additional sessions may be desired.6
These goals should be the ultimate aim of those performing hair restoration surgery, but may not be achievable with a small or inexperienced surgical team. In this author’s opinion, there is little medical or aesthetic justification for performing the surgery in arbitrarily small sessions. It is preferable that each procedure cover the entire area of hair loss intended to be treated and be designed to “stand-alone.”6,7
In an alternate approach to surgical planning, the objective is to achieve final density in a one-pass procedure by creating high density in a more limited area and then transplanting another area in a subsequent session. This is accomplished in part by using very small recipient sites, limiting graft size to three hairs and using the stick-and-place method, in which grafts are inserted as soon as the recipient sites are made. ((Seager D. The “one-pass hair transplant” – a six-year perspective. Hair Transplant Forum Int 2003;12:176–8, 194–196.))
As the number of grafts placed per unit area (density) rises, so too does the risk of vascular compromise that may result in sub-optimal graft growth. Technical problems of popping that may cause the grafts to become desiccated or that expose the grafts to mechanical injury on reinsertion also become more likely. Proper patient selection and technical expertise help avoid such problems, but the risks are increased nevertheless. It certainly behooves those with more limited experience to be conservative when determining the number of grafts to be transplanted.
Because the blood supply to the scalp is extensively collateralized, ((Salasche SJ, Bernstein G, Senkarik M. Surgical anatomy of the skin. Norwalk, CT: Appleton & Lange; 1988. p. 151–62.)) the risk of vascular compromise is related more to the density of grafts in a specific area and the size of the recipient wounds, than the absolute numbers of grafts placed. For this reason, the transplantation of a large number of grafts over a broad area does not seem to pose the same problems as producing very high densities in specific areas. In addition, popping becomes less of a technical issue when the same numbers of grafts are placed over a larger area. However, transplanting large number of grafts (in “mega-sessions”) poses its own challenges, such as increasing the time the grafts remain outside the body, requiring more staff, contributing to patient and staff fatigue, and creating organizational issues. As with dense packing, the use of very large sessions should be reserved for only the most experienced surgical teams.
Though the amount of hair needed to cover the front and top of the patient’s scalp will vary, an attempt should be made to achieve this coverage, if only lightly, in the first session. Crown coverage should not be a goal of the first session unless the patient has an above-average donor supply; if it is attempted in the first session, the patient’s options will be more limited and the chances for an aesthetically balanced transplant may be permanently compromised.
Table 30.2 offers general guidelines as to the number of follicular units recommended for the first hair transplant procedure.
| Table 30.2 Number of grafts in first follicular unit transplant session | ||
| Norwood Class | Follicular Units | Total Units with crown |
|---|---|---|
| IIa | 800-1400 | – |
| III | 1000-1600 | – |
| III Vertex | 1200-1600 | 1600-2200 |
| IIIa | 1400-1800 | – |
| IV | 1600-2200 | 2200-2600 |
| IVa | 1800-2400 | – |
| V | 2000-2500 | 2500-2800 |
| Va | 2200-2800 | – |
| VI | 2400-3000 | 2800-3400 |
| VII | 2500-3200 | 3000-3600 |
Planning a second session
Timing of the second session
Transplanted hair sheds around 2–6 weeks after the procedure and the first signs of new growth occur on average in approximately 10 weeks, though the onset can vary considerably, taking as long as 4–6 months or more. Hair gradually increases in both thickness and in length, so that the initial growth is often not indicative of the final result. In less than 5% of patients, hair growth proceeds unabated after surgery, without shedding.
One should generally wait 10–12 months to best appreciate the cosmetic impact of the procedure. During this time, the continued increase in the diameter and length of the newly growing hair strikingly alters the appearance of the restoration. Once hair reaches styling length, both the patient and physician can make aesthetic judgments about the desirability of an additional treatment session. It will also be advantageous to delay the second procedure to maximize the donor harvest. Although each procedure results in a potentially tighter scalp, some of the preoperative donor laxity returns in the months following the surgery. Although, the major change will occur during the first month as the edema and inflammation subside, further loosening occurs as the scalp stretches over the next 12 months.
In the uncommon event of telogen effluvium occurring in the donor area, the telogen follicles may not be easily identifiable in the dissection. Since recovery in the donor area may take up to a year, it is essential that patients wait until complete regrowth has taken place before a second session is performed. In rare cases, if the closure is too tight, the effluvium may eventuate in permanent hair loss.
Goals for the second session
The main goals of the second session are:
- To add density to areas transplanted in the first session
- To refine the hairline
- To follow the progression of the hair loss (if necessary)
- To extend the transplant into the crown (when appropriate)
- To complement a specific grooming pattern
At 1-year follow up, approximately 50% of patients are satisfied with the density achieved in the first session. However, if the first session is planned properly (as a stand-alone procedure) it should always look natural, regardless of the desire for more fullness. This gives the person who may want further density to perform the next procedure at his or her leisure, or not at all.
The hairline can sometimes benefit from a little fine- tuning to make the leading edge softer and more irregular. Placing the hairline too low is a common mistake, but so is placing it too high, where it fails to frame the face. One should avoid transplanting a hairline with the intention of bringing it down on the second session, once the patient can see how it looks. It is better to try to get the hairline position right in the first session; lowering it later often leaves the leading edge too thin and may necessitate an additional, otherwise unnecessary procedure.
The decision regarding crown coverage is important because it is the least visible of the balding regions, but can potentially occupy a very large surface area, producing an almost inexhaustible demand upon the donor supply.
If extensive balding appears likely and the patient has a modest donor supply, the crown should be treated only by extending the transplant of the top of the scalp posteriorly, rather than by transplanting the crown as an isolated region. This will help to ensure that the patient will not be short of hair if the intervening bridge between the front and crown were to require additional grafts. Unless the patient’s history, age, and physical examination indicate limited balding, it is particularly important to avoid creating significant density in the crown, as subsequent balding could leave an isolated island of hair.
A useful alternative to covering the crown with trans- planted hair is to end the transplant at the vertex transition point (the curved line that delineates the area where hair changes from a predominantly forward direction into a swirl).39, ((Beehner M. Where is thy crown, your majesty? Hair Transplant Forum Int 1998;8:18–19.)) The patient can then groom the forward-pointing hair back to conceal the non-transplanted area. This is recommended when direct crown coverage is not realistic, or it is too early in the balding process to determine whether significant crown coverage will be possible in the future. Another advantage of sparing, or lightly covering, the crown is that donor reserves may be saved to address possible further diminution of the lateral donor fringe, which would separate the transplanted hair on the top of the scalp from the sides. Connecting these two areas is generally more important cosmetically than covering the crown.
Particularly when a person has been bald for many years, it is not known how the person will want to style his or her hair after the restoration. Once the first procedure has grown in, many people will feel comfortable committing to a specific grooming pattern. If this is the case, then the second procedure may be used to complement a specific way of styling the hair. For example, if a man is relatively certain that he will continue to comb his hair from left to right, then the transplant can be left-weighted to enhance the appearance of density when the hair is groomed in this manner. If, however, the person prefers to comb his hair straight back, or wants to vary his look, then the second transplant should be symmetrical, mimicking the distribution of the first.
Subsequent transplant sessions
The surgeon should make every attempt to accomplish the restoration in as few sessions as possible, rather than putting patients through an unnecessarily protracted course of multiple surgeries that can adversely affect their donor area and their lifestyle. The number of grafts required to achieve patient satisfaction varies widely due to the great variability in hair characteristics and patient tastes. Moreover, because hair loss patterns form a continuum, the number of grafts necessary for each discrete Norwood class can vary significantly. Table 30.3 shows the approximate number of follicular unit grafts necessary for a complete restoration, with and without crown coverage.
| Table 30.3 Total number of follicular unit grafts for complete restoration | ||
| Norwood Class | Follicular Units | Total units with crown |
|---|---|---|
| IIa | 1400-2200 | – |
| III | 1600-2400 | – |
| III Vertex | 1800-2600 | 2600-3200 |
| IIIa | 2000-3000 | – |
| IV | 2200-3400 | 3400-3800 |
| IVa | 2400-3600 | – |
| V | 2600-3800 | 3800-4500 |
| Va | 2800-4200 | – |
| VI | 3000-4600 | 4600-5600 |
| VII | 3200-5000 | 5000-6400 |
The patient
It is common practice for doctors to place patients on finasteride before surgery to minimize the chance of postsurgical effluvium (shedding). Although there are no scientific studies confirming that it is effective for this purpose, it would seem that if the goal is to prevent post-surgical shedding, the medication should be prescribed at least a month before the procedure. If, however, the intent is to postpone or obviate the need for surgery, then it should be taken for a minimum of 1 year to allow sufficient time for the patient’s hair to regrow.
Patients using topical minoxidil are advised to discontinue its use several days before surgery (because of its vasodilator properties) and wait until a week after the procedure before resuming it (to avoid the irritating effects of the alcohol in the 2% solution or the propylene glycol in the 5% solution). Although we suggest minoxidil in combination with finasteride for patients with early hair loss, we generally do not recommend the use of minoxidil after a hair transplant, unless it is being used in an area other than that which was transplanted (such as the crown). We feel that minoxidil has little added value for the average post-transplant patient. We do, however, encourage the continued use of finasteride to help retard further balding. Those who do not plan to use minoxidil after surgery should discontinue it immediately, so that any of its beneficial effects may be reversed by the time of surgery and the true extent of a person’s hair loss be more easily appreciated.
Patients are advised to avoid products that are used to thicken the hair or that stain the scalp for 3 days before the procedure, as these often take several days to completely wash out of the scalp. Their presence during surgery can decrease visibility and make placing more difficult. Hair systems should be removed before surgery and be replaced with, or converted to, a clip-on system. The front edge of the piece can be kept in place with a stiffening rod, obviating the need for tape or glue. However, any form of attachment at or near the frontal hairline risks dislodging grafts. Patients are encouraged to permanently discontinue the system after the procedure, but those who feel the need to use them until their hair grows in should wait at least 10 days postoperatively when the grafts are secure.
| Box 30.3 Preoperative instructions |
|
Systemic antibiotics are not indicated for clean surgical wounds in healthy patients, so their routine use in hair transplantation is not necessary; however, many doctors routinely use them. Because there are no specific guidelines for antibiotic prophylaxis in hair transplantation for patients with a history of endocarditis or mitral valve prolapse, the decision to use them must be based on the individual patient’s risk factors. ((Haas A, Grekin R. Antibiotic prophylaxis in dermatologic surgery. J Am Acad Dermatol 1995;32:155–76.))
Other preoperative instructions are relatively straightforward and will depend to some degree on the preferences of the operating surgeon. Patients should be notified well in advance of the procedure date regarding the need to discontinue certain medications, to stop smoking, and abstain from alcohol. Box 30.3 is a summary of the main preoperative instructions.
All patients undergoing hair transplantation should be treated with universal precautions. Although there is no consensus on the need to perform routine blood tests before the procedure, the following tests provide an additional level of safety:
- Complete blood count
- Metabolic panel
- Hepatitis B surface antigen and antibody
- Hepatitis C antibody
- HIV screen.
| Box 30.4 Elements of consent form for hair transplant |
|
One should obtain medical clearance for HIV-positive patients to make sure that they are immunocompetent enough to withstand a potential series of procedures. With hepatitis antigen-positivity, one should inform the patient’s primary physician to rule out active disease. Other tests, such as a platelet count and prothrombin and thromboplastin times, are performed only if warranted by the history and physical exam.
The surgical consent form should be given to patients to read at their leisure well in advance of the procedure and should be signed by the patient before taking any medications that can cause drowsiness or impair judgment. The exact time of signing should be indicated. The major elements of the consent form are listed in Box 30.4.
Although not universally recommended, some doctors have the patient shower the morning of surgery using a chlorhexidine surgical scrub as shampoo. Though it does not sterilize the scalp, the chlorhexidine binds to the stratum corneum, decreasing transient and pathogenic microorganisms and resident skin flora. Caution is advised as it can be toxic to the middle ear and irritating to the eyes. In our practice, we advise patients to use a simple baby shampoo. After showering, the patient should dress in comfortable clothes and wear a button-down shirt.
THE OPERATING ROOM
Follicular unit hair transplantation is typically performed in an outpatient office setting. The guidelines of care for office surgical facilities delineated by the American Academy of Dermatology should be reviewed before setting up facilities. ((Drake LA, Ceilley RI, Cornelison RL, et al. Guidelines of care for office surgical facilities, part I. J Am Acad Dermatol 1992;26:763–5.)), ((Drake LA, Ceilley RI, Cornelison RL, et al. Guidelines of care for office surgical facilities, part II. Self-assessment checklist. J Am Acad Dermatol 1995;33:265–70.)) Though major complications occur only rarely, protocols should be in place to handle emergencies such as hypersensitivity reactions or anaphylaxis, stroke, seizures, arrhythmias, acute myocardial infarction, and hypertensive crisis. Specific arrangements should be made with a local Emergency Medical Services (EMS) facility to take care of distressed patients. ((Fader DJ, Johnson TM. Medical issues and emergencies in the dermatology office. J Am Acad Dermatol 1997;36:1–16.))
The medical staff should be comfortable dealing with problems such as bleeding, syncope, petit mal episodes, and anxiety reactions. The medical staff should be certified in cardiopulmonary resuscitation (CPR), and at least some clinical staff in advanced cardiac life support (ACLS). The amount of in-office emergency equipment will depend on staff capability and training, and proximity to an EMS unit. Basic equipment includes portable oxygen, an automated defibrillator, intravenous set-up, and an oral and nasopharyngeal airway.46
Because of the scalp’s abundant vascular supply and relative resistance to infection, it is common practice for doctors’ technique to be aseptic, rather than sterile. And because the scalp is not usually shaved and prepped, strict sterile technique is not practical. However, at least until the donor area is sutured closed, a sterile environment should be maintained. This issue is discussed in greater detail by Sebben. ((Sebben JE. Survey of sterile technique used by dermatologic surgeons. J Am Acad Dermatol 1988;18:1107–12.)), ((Sebben JE. Sterile technique in dermatologic surgery: what is enough? J Dermatol Surg Oncol 1988;14:487–9.))
The single most important instrument for FUT is the dissecting stereomicroscope,8, ((Bernstein RM, Rassman WR. Dissecting microscope versus magnifying loupes with transillumination in the preparation of follicular unit grafts: a bilateral controlled study. Dermatol Surg 1998;24:875–80.)) which is available with either a binocular view or an LCD screen. There should be one microscope for each member of the surgical team performing dissection. Experienced teams generally require one staff member per 500–750 grafts (for graft dissection and placing), so that a typical 2000-graft procedure would necessitate three or four staff members in addition to the physician, though this will vary considerably among practices. Less experienced teams may require more people. The staff’s other office responsibilities, as well as possible absenteeism, must also be taken into account in calculating the number needed. The staffing requirements for FUE are somewhat smaller.
Regular operating room tables are generally inadequate. Rather, the tables must be contoured and provide some lumbar support when patients are sitting. They also need to be low to the ground, with the seat not more than 56 cm from the floor, so the staff can work comfortably around the head, while the patient is seated.
Equally important are comfortable working areas for the staff. Dissecting tables with a bull nose, rather than squared edge, are easier on their arms, and adjustable seating saves them from bending over to look into the microscopes (Fig. 30.5). The long duration of the surgery makes meticulous attention to ergonomic issues all the more crucial. ((Blugerman G, Schavelzon D. Ergonomics applied to hair restoration. Hair Transplant Forum Int 1996;6:1–14.)) Bright fluorescent ceiling lighting is preferable to surgical operating room lights because it generates less heat. We use high intensity surgical lights only for working on the patient’s donor area, as they can be angled to illuminate the posterior scalp of the seated patient. ((Bernstein RM, Rassman WR. Limiting epinephrine (adrenaline) in large hair transplant sessions. Hair Transplant Forum Int 2000;10: 39–42.))
Petri dishes containing grafts pending placement should be kept on a stable, wall-mounted surface to keep them from being inadvertently knocked over (Fig. 30.6). The typical operating room Mayo stand is not sufficiently stable. ((Bernstein RM, Rassman WR. Wall mounted placing stand. Hair Transplant Forum Int 1997;7:17–18.)) Normal saline is the most frequently used holding solution for grafts. Limmer has shown a high survival rate for grafts kept in chilled saline for up to eight hours. ((Limmer BL. Micrograft survival. In: Stough DB, Haber RS, editors. Hair replacement: surgical and medical. St Louis: Mosby; 1996. p. 147–9.)) More exotic solutions with potential graft-sustaining properties, such as platelet-rich plasma or those containing ATP, have been developed, but they are not yet widely used. ((Raposio E, Cella A, Panarese P, et al. Power boosting the grafts in hair transplantation surgery. Evaluation of a new storage medium. Dermatol Surg 1998;24:1342–6.)), ((Krugluger W, Moser K, Hugeneck J, et al. New storage buffers for micrografts enhance graft survival and clinical outcome in hair restoration surgery. Hair Transplant Forum Int 2003;13:325,333–334, 343.)), ((Greco J, Brandt RJ. Preliminary experience and extended applications for the use of autologous platelet-rich plasma in hair transplantation surgery. Hair Transplant Forum Int 2007;17:131–2.))
 FIGURE 30.5 Dissecting stereomicroscopes on cutting table.
FIGURE 30.5 Dissecting stereomicroscopes on cutting table. FIGURE 30.6 Wall-mounted placing stand for ice blocks, grafts, and instruments.
FIGURE 30.6 Wall-mounted placing stand for ice blocks, grafts, and instruments.(From Bernstein RM, Rassman WR. Wall mounted placing stand. Hair Transplant Forum Int 1997; 7:17–18.)
Although it is standard practice to chill grafts in order to slow down cellular metabolism and increase their survival outside the body, there is concern that isotonic holding solutions (such as normal saline and lactated ringers) may not be ideal for this cooled environment. At temperatures used for hypothermic storage (2–8°C) the ATP-driven ion pumps that maintain osmotic balance are inactivated, allowing salt and then water influx. This can result in intracellular edema and cell death.
New holding solutions, that contain impermeant macromolecules which mimic the intracellular environment of grafts, can prevent fluid influx at cooler temperatures. Antioxidants that decrease free radical formation and buffers that keep the ph in an optimal range can also be added to the mix in order to increase the survival of transplanted grafts.
It is preferable to have all the dissection performed in the operating room. A small cooling station for grafts should be located between every two dissectors. Performing all the dissection and storing the grafts in the same room eliminates the risk of inadvertently placing grafts in the wrong patient. This is particularly important in a busy practice where more than one person is being operated on per day. The minimum room size for a physician and five staff members to work comfortably is approximately 15.5 square meters.
In advance of the procedure, it is helpful to set up complete surgical trays, including the local anesthetic mixtures pre-drawn into syringes. This serves a number of purposes: it makes it easier to keep track of how much anesthetic is used in each part of the procedure, it helps to ensure that safe dose limits are not exceeded, ((Grekin R, Auletta M. Local anesthesia in dermatologic surgery. Am Acad Dermatol 1988;19:599–614.)) and it minimizes the risk of needle-stick injury, because the preparation takes place before the operating room begins to bustle.
Local anesthesia is administered with a ring block consisting of lidocaine, bupivacaine, and epinephrine buffered with sodium bicarbonate. Lidocaine, the major component, is used for its safety and quick onset. Bupivacaine is added to increase the duration of anesthesia, but in smaller quantities to limit its potential cardiac toxicity. ((McCaughey W. Adverse effects of local anesthetics. Drug Safety 1992;7:178–89.)) Epinephrine increases the anesthetic duration while decreasing its toxicity and providing some hemostasis – although its vasoconstrictive action is relatively short lived.
Sodium bicarbonate can be added to bring the acidic pH of the epinephrine-containing solution closer to 7.4, lessening its sting. ((Skidmore R, Patterson J, Tomsick R. Local anesthetics. Dermatol Surg 1996;22:520.)) This is particularly useful for injections at the hairline, where the scalp tends to be most sensitive. It is important to stress that the combined use of epinephrine and broad β-blockers (e.g., propranolol) can result in potentially life-threatening reactions. ((Foster C, Aston S. Propranolol–epinephrine (adrenaline) interaction: a potential disaster. Plast Reconstr Surg 1983;72:74–8.))
The anesthetic solution used for a typical 2000-graft session is listed in Table 30.4. The anesthetic is drawn into 13 5 mL syringes then fitted with 27-gauge needles. The tumescent mixture consists of three 10 mL syringes with 25-gauge needles. These quantities are pre-printed on the operative report and circled as each syringe is used. If the patient requires additional anesthesia, the medication is drawn up and the additional doses are recorded.
| Table 30.4 Anesthetic mixture | ||
| Ring block | ||
| Component | Concentration | Amount in mixture |
| Lidocaine | 0.50% | ≈60% |
| Bupivacaine | 0.25% | ≈40% |
| Sodium bicarbonatea | 8.40% | 1 : 10 |
| Epinephrine | – | 1 : 200 000 |
| Volumeb | Initial quantity | Boost |
| Donor area | 20 cc | 10 mL |
| Recipient area | 25 cc | 10 mL |
| Tumescent mixture for donor harvest | ||
| Component | Concentration | Amount in mixture |
| Lidocaine | 0.17% | ≈100% |
| Epinephrine | – | 1 : 600 000 |
| Volumeb | Quantity | |
| Donor area | 30 cc | |
| aUsed prn. bVolume of mixture typically used in 2400-graft session. |
||
TECHNIQUES
On arriving at the office, patients sign the consent form which was given to them well in advance. The surgical plan is reviewed and any last-minute questions are answered. Next, density is checked to confirm the measurement taken at the initial consultation. If the number differs or it appears that density varies in the donor area, multiple measurements are taken. Scalp laxity is also reassessed and recorded on the operative report.
Patients are given cotton surgical gowns to wear and their pictures are taken with a digital camera against a light-blue background. Next, the hairline and other important landmarks, such as the vertex transition point and crown swirl, are marked in gentian violet and shown to the patient using two mirrors. If the patient approves of the hairline design, additional pictures are taken, this time of the marked scalp, usually from the front, top and posterior views. Three-quarter and close-up photographs are taken to illustrate particular cosmetic issues. Occasionally, pictures are taken during the operation for teaching purposes. At least one photograph is taken at the end of the procedure. All photographs are kept as part of patients’ permanent medical records.
Typically, for a procedure of 1500 grafts or more, patients are pre-medicated with oral diazepam 5–15 mg and intramuscular midazolam 2–4 mg about 15 min prior to surgery.
Dosing will depend upon the patient’s body weight, age, sex, and past sedative/analgesic use. Sedatives are not given to patients who will be driving themselves home from the procedure. Intramuscular methylprednisolone 80 mg is given to reduce postoperative swelling. Oral antibiotics are not routinely given, but are considered in patients with implanted prosthetic devices, valvular heart disease, and other medical conditions requiring prophylaxis.
Donor harvest
In preparation for the donor harvest, patients are seated upright on the operating table. The hair in the donor area (hair in the proposed donor strip plus 0.5 cm above and below the strip to facilitate suturing or stapling) is then cut to a length of 2 mm using electric clippers. The trimmings are thoroughly vacuumed away and the hair above the strip is held back with paper tape, fully exposing the donor area. A gauze headband is placed around the patient’s head just below the clipped area and sterile drapes are taped to the headband.
In the first session of FUT, in a patient with average scalp laxity, we generally harvest a donor strip that is 1.2–1.4 cm wide. In calculating the length, we use the general rule of 70–95 follicular units/cm2.5 For example, if a 2000-follicular unit graft hair transplant is planned for a Caucasian male with an average density of 85 FU/cm2, a 1.2 cm-wide strip would need to be about 20 cm in length (plus tapered corners to allow a flat closure). People of African and Asian descent characteristically have a lower follicular unit density and a strip of this dimension will yield a lower number of follicular unit grafts.7
When performing follicular unit extraction, one can generally extract about 18 FU/cm2, so the surgeon would need about five times the area of an FUT strip to get the equivalent number of grafts. Thus, to extract the 2000 grafts in the above example, one would need an area measuring 1.2 × 20 × 5 cm or 120 cm2. In FUE, the hair should be clipped to a length of 1 mm (half the length of the 2 mm needed for FUT).
Local anesthesia
A ring block is established using an anesthetic mixture of lidocaine, bupivacaine, and epinephrine (see Table 30.4). The anesthetic solution is injected into the subcutaneous fat layer approximately 1 cm below the lower portion of the clipped area and extending several centimeters past it on either side. In addition to buffering the anesthetic, already discussed, discomfort from the injections can be reduced by pressing a vibrating hand massager to the skin below the area of insertion of the needle. The sting of the injection can also be reduced by a device that controls the flow of the anesthetic solution. ((Hochman MN, Chiarello D, Hochman CB, et al. Computerized local anesthetic delivery vs. traditional syringe technique: subjective pain response. NY State Dent J 1997;63:24–9.))
Approximately 0.75 mL of anesthesia is used per centimeter of donor area, so that a 25 cm long incision would require slightly less than 20 mL of anesthetic solution. It is important to avoid injecting into the muscle, as epinephrine will cause vasodilatation, due to the action on β2-receptors, quickly dissipating the local effects of the anesthetic and increasing its toxicity. ((Goodman AG, Gillman LS. The pharmacological basis of therapeutics. 7th ed. New York: Macmillan; 1985. p. 151–9.))
The ring block takes approximately 15 min to induce anesthesia. As soon as the donor-area skin becomes numb, tumescent anesthesia is administered by injecting larger quantities of a more dilute lidocaine–epinephrine solution into the mid-fat to make the area firm. The tumescence serves six purposes: (1) to widen the distance from the follicles residing in the upper fat to the nerves and larger blood vessels lying just above the fascia; (2) to increase the rigidity of the donor area; (3) to decrease follicular transection; (4) to decrease bleeding; (5) to produce more uniform anesthesia; and (6) to reduce the amount of anesthetic required.
Tumescence can be achieved using a dilute solution of epinephrine and lidocaine (see Table 30.4). This will provide additional anesthesia that is particularly important in repeat procedures, or for patients with significant donor scarring. ((Bernstein RM, Rassman WR. Hemostasis with minimal epinephrine. Hair Transplant Forum Int 1999;9:153.)) In situations where there is prior surgery, direct cutaneous innervation to the donor area from the occipital branches can be blocked by scar tissue, so that innervation to the donor area arrives in a rostral–caudal direction, rendering inferiorly placed ring block anesthesia alone ineffective. At times, when there is an excessive amount of donor scarring, the anesthetic mixture must be injected above or directly into the scarred area to make it numb.
Strip excision
 FIGURE 30.7 Donor-strip harvesting using two parallel blades on a Rassman handle pre-agled at 30° to allow both blades to lie flush against the scalp.
FIGURE 30.7 Donor-strip harvesting using two parallel blades on a Rassman handle pre-agled at 30° to allow both blades to lie flush against the scalp. FIGURE 30.8 Dissection at the tapered corner of the strip in a mid-subcutaneous plane, just below the follicles.
FIGURE 30.8 Dissection at the tapered corner of the strip in a mid-subcutaneous plane, just below the follicles.The ideal position for the donor incision is in the midportion of the permanent zone. This generally lies at the level of the external occipital protuberance (at the midline). The donor strip can be excised using two parallel blades set 1.0–1.5 cm apart or the strip can be harvested using a single blade. When using the parallel blade technique, a convenient harvesting device is the Rassman handle that is loaded with two no.10 blades. The handle holds the blades at an angle of 30°, making them parallel to the emergent hair. The handle should hold the blades in a pre-angled position to allow both blades to lie flush with the skin surface when aligned with the hair follicles; otherwise, the surgeon may cut too superficially with the upper blade and too deep into the scalp with the lower (Fig. 30.7). Once the main parallel portion of the incision is complete, a single scalpel blade is used to taper the ends into an ellipse and to dissect the base of the strip in mid-fat, just below the level of the follicles. In general, the length of each tapered end should measure at least 1.5 times the width of the strip, so that the ends lie flat (Fig. 30.8).
Alternatively, the skin can be marked and the entire excision can be made with a long free-hand ellipse.8 This has the advantage of allowing the angle of the blade to be adjusted to the angle of the follicles as each edge is cut. However, this technique makes it harder to keep the width uniform, which is necessary for predictable graft yields. In addition, the first incision causes the strip to lose its rigidity, making the cutting the second edge more difficult Regardless of the harvesting method, once the strip is removed it is immediately placed into a holding solution.
Donor closure
 FIGURE 30.9 Stapling technique illustrating very controlled opposition of wound edges, using skin hooks for the lower edge, and forceps to evert the upper.
FIGURE 30.9 Stapling technique illustrating very controlled opposition of wound edges, using skin hooks for the lower edge, and forceps to evert the upper.(From Bernstein RM, Rassman WR, Rashid N. A new suture for hair transplantation: poliglecaprone 25. Dermatol Surg 2001; 27:5–11.)
 FIGURE 30.10 Suturing the donor area using absorbable sutures. Note the needle track is parallel and very close to the wound edges.
FIGURE 30.10 Suturing the donor area using absorbable sutures. Note the needle track is parallel and very close to the wound edges.(From Bernstein RM, Rassman WR, Rashid N. A new suture for hair transplantation: poliglecaprone 25. Dermatol Surg 2001; 27:5–11.)
 FIGURE 30.11 Schematic of the suturing technique recommended for closing the donor scalp. The spacing between loops is 4.5mm.
FIGURE 30.11 Schematic of the suturing technique recommended for closing the donor scalp. The spacing between loops is 4.5mm.(From Bernstein RM, Rassman WR, Rashid N. A new suture for hair transplantation: poliglecaprone 25. Dermatol Surg 2001; 27:5–11.)
The most common types of closures include metal staples, a running non-absorbable suture, or a running absorbable suture. ((Bernstein RM, Rassman WR, Rashid N. A new suture for hair transplantation: poliglecaprone 25. Dermatol Surg 2001;27:5–11.)), ((Bennett RG. Selection of wound closure materials. J Am Acad Dermatol 1988;18:619–37.)), ((Moy RL, Waldman B, Hein DW. A review of sutures and suturing techniques. J Dermatol Surg Oncol 1992;18:785–95.)) If staples are used to close the wound edges, it is important to be certain that the wound edges are flush before the staples are applied. The edges can be approximated by grasping the lower edge with a skin hook and using rat-toothed forceps to hold and slightly evert the upper edge (this requires the help of an assistant) (Fig. 30.9). The staples should then be placed approximately 0.6 cm apart. Staples have very little tissue reactivity, but they make flush apposition of wound edges a little more difficult and can be uncomfortable in the postoperative period.64 In the author’s practice, we routinely remove alternating staples at 10 days and the remainder at 18–25 days postoperatively, depending on the degree of tension, the patient’s age and the amount of anticipated activity. We feel that leaving in staples for this longer period of time leads to a finer donor scar, particularly in younger or active patients and in those with tighter than average closures.
Non-absorbable sutures, of either nylon or polypropylene, are usually placed using a single running stitch and are removed in 10–14 days. A problem is that if they are placed too far from the wound edge, they entrap hair follicles and this may result in the destruction of donor hair if there is significant postoperative edema. If placed too close to the wound edge, they can become buried and difficult to remove. These two problems are avoided with the use of staples and probably speak to why staples are used by so many hair transplant surgeons in spite of the preference that patients have for the more comfortable sutured closure.
| Box 30.5 Guidelines for use of poliglecaprone 25 sutures |
|
Absorbable sutures should be placed close to the wound edge (Fig. 30.10). This will tend to minimize the amount of suture material used, decrease the entrapment of follicles and avoid strangulation if there is significant postoperative edema. To further limit damage to follicles, the sutures should be placed approximately 4.5 mm apart and the suture should be advanced on the surface, rather than under the skin, as this will minimize the amount of suture in contact with the follicles (Fig. 30.11). With this technique, the needle should be passed through the full thickness of the dermis and exit the wound edge, just below the level of the bulbs, without incorporating any significant amount of subcutaneous tissue. The needle track must be kept parallel to, and within, 1.5 mm of the wound edge. Particular attention should be paid to placing the needle parallel to the upper wound edge where the angle is very acute. Although hypoallergenic, in our experience, poliglecaprone can cause allergic reactions in some patients. Basic guidelines for using poliglecaprone 25 sutures can be found in Box 30.5.
Graft dissection
Once the donor strip is removed, it is immediately placed into the holding solution and passed to the surgical team leader stationed at a stereomicroscope (Fig. 30.12). The strip dimensions are measured and recorded. The next step is performed under strict stereomicroscopic control in a process called “slivering.”27,40
 FIGURE 30.12 Close-up view of a donor strip showing the natural hair groupings at the surface, the slightly curved path of the hair throught the dermis, and the random distributions of bulbs in the fat. It is this unique anatomy that makes stereomicroscope dissection essential in preventing follicular transection.
FIGURE 30.12 Close-up view of a donor strip showing the natural hair groupings at the surface, the slightly curved path of the hair throught the dermis, and the random distributions of bulbs in the fat. It is this unique anatomy that makes stereomicroscope dissection essential in preventing follicular transection. FIGURE 30.13 “Silvering” the donor strip into sections.
FIGURE 30.13 “Silvering” the donor strip into sections. FIGURE 30.14 Stereomicroscopic dissection of one section into smaller pieces.
FIGURE 30.14 Stereomicroscopic dissection of one section into smaller pieces. FIGURE 30.15 The final step of the dissection: generating individual follicular units.
FIGURE 30.15 The final step of the dissection: generating individual follicular units. FIGURE 30.16 Dissected one-hair, two-hair, three-hair, and four-hair follicular units.
FIGURE 30.16 Dissected one-hair, two-hair, three-hair, and four-hair follicular units.In one method of slivering, the donor strip is placed on its side, on a wooden tongue-depressor blade, soaked in Ringer’s, with the hair pointing away from the dissector and the convex surface of the strip facing upward. For a right-handed person performing dissection, the left end of the strip is held with rat-toothed forceps in the dissector’s left hand. An assistant applies tension to the strip, holding it a few centimeters away from the area being cut with rat-toothed forceps held in the right hand. Using a Persona no.10 blade on a no.3 blade handle-held in the right hand, the dissector begins to cut the strip into 2–2.5 mm-wide sections, by guiding the blade between follicular units using a one-directional fillet-like movement from the epidermal side to the subcutaneous surface. A back-and-forth sawing motion should be avoided, as it will increase the risk of transection (Figs 30.13, 30.14). The pieces generated are then distributed to the other members of the surgical team who complete the dissection. In the last step, the individual pieces are placed on their sides, stabilized with straight jeweler’s forceps, and then dissected under the stereomicroscope with a scalpel into individual follicular units of one to four hairs each (Figs 30.15, 30.16).
Next, the units are sorted according to the number of hairs they contain and placed into Petri dishes with 4-quadrant dividers to hold separate the different size follicular units. The Pitri dishes containing holding solution, are placed on cooling unit to keep the solution at a specified temperature (Fig. 30.17). The temperature is monitored with a digital sensing device.
 FIGURE 30.17 Follicular units in holding solution on a cooling unit. The temperature is monitored with a digital sensing device.
FIGURE 30.17 Follicular units in holding solution on a cooling unit. The temperature is monitored with a digital sensing device.An alternate slivering method involves dissecting the donor strip into slivers as wide as one follicular unit, approximately 1 mm. The units are then isolated from the very thin strip. The key to either method is that every step is performed under stereomicroscopic control, which keeps the units intact and avoids follicular transection. It is also vital that all the pieces of the strip remain in chilled Ringer’s lactate, except when they are being cut. While under the microscope they should be kept well hydrated using 10 mL syringes containing Ringer’s lactate that is kept at each cutting station.
Follicular unit extraction
Follicular unit extraction (FUE)10 is a conceptually simple procedure, where individual follicular units are harvested through small circular incisions created by a trephine or similar round instrument. In FUE, the punch cuts down through the reticular dermis and the follicular unit is pulled (extracted) from the scalp. The advantage of this procedure is that it obviates the need for a linear incision. Difficulties lie in the increased risk of follicular transection compared to strip harvesting, the inability to harvest all the hair from the mid-portion of the donor area, and the intrinsic variability of each patient with respect to the ease of extraction. There are also the organizational challenges of not being able to have multiple persons working in parallel (as can be done with the microscopic dissection of a harvested strip) and the inability to easily harvest and place grafts at the same time. Both of these constraints serve to increase the length of the FUE procedure.
The main indication for FUE is for patients who want to wear their hair so short that the linear donor scar of FUT may become visible. FUE is also useful in patients who are at risk to having a widened donor scar. This includes very athletic patients, particularly those engaged in contact sports, bodybuilders, and younger patients in general. Other indications include patients with very tight scalps (in whom a strip excision is not practical) and those with a history of healing poorly, i.e., those that heal with stretched or hypertrophic scars, or with keloids. Some patients simply prefer not to have a linear donor scar.
An important application of FUE is to camouflage a widened linear donor scar from prior hair transplant procedures.10 As stated earlier in this chapter, it is unfortunate that patients with very loose scalps are at risk of healing with widened scars but may also be poor candidates for FUE due to a difficulty in adequately stabilizing the scalp.38
FUE’s main limitation is that it is less efficient in harvesting hair than FUT performed with strip harvesting. In FUT, all the hair from the optimal (central) part of the donor region can be removed and transplanted, and the resulting defect is sewn closed. In contrast, the FUE defects remain open to heal by second intention with significant amounts of intervening hair left behind and, therefore, a much larger region of the scalp must be accessed to harvest the necessary amount of donor hair. In a single procedure, to obtain the equivalent number of grafts, FUE requires approximately five times the area needed when using a strip. In FUE, the greater area required significantly increases the risk of harvesting hair from non-permanent areas of the scalp or from areas where the hair is of poorer quality. Figure 30.18 shows the donor area immediately after the extraction of 1891 follicular unit grafts and the same area 1- and 2-weeks postoperatively.
As mentioned, transection during the harvest is greater in FUE than with stereomicroscopically controlled FUT. This is due to the inability of the instruments used in FUE to fully account for the variable angles and curved path of the individual follicles that comprise each follicular unit as they pass deeper in the skin. With FUE, the second intention healing causes microscopic fibrotic changes in the donor area, distorting adjacent follicular units and making subsequent sessions more difficult. Taken together, these factors limit the total amount of intact follicles that can be harvested through FUE, rendering it a less robust procedure than FUT. In a procedure where a finite donor supply is the main constraint, the inefficient use of donor tissue poses a significant problem. Table 30.5 summarizes the pros and cons of FUE.
The ability to perform FUE with minimal transection varies significantly among patients and in different regions of the scalp. Because of this, a test measuring the ease of extraction (the FOX Test)10 should be performed prior to recommending the procedure in patients where extraction is likely to be difficult. Patients undergoing FUT can easily be tested for FUE at the time of surgery in case the latter procedure is considered at a future date.
A significant advance in the technique of FUE is to first score the top of the follicular unit with a sharp punch and then use a blunt instrument to separate the follicular unit from the surrounding tissue, thereby minimizing follicular transection. The main limitations of this technique are the increased incidence of buried grafts and increased surgical time in an already very laborious process. ((Harris JA. The SAFE system: new instrumentation and methodology to improve follicular unit extraction. Hair Transplant Forum Int 2004;14:157, 163–164.)), ((Bernstein RM. New instrumentation for three-step follicular unit extraction. Hair Transplant Forum Int 2006;16:229, 237–239.)) Robotic FUE, based on this two-step concept, addresses the limitations of conventional techniques. ((Canales MG. The age of surgical robotics. Hair Transplant Forum Int 2008;18:95–6.))



FIGURE 30.18 (A) Donor area in follicular unit extraction immediately after harvesting 1891 grafts. (B) After 1 week postoperatively. (C) At 2 weeks postoperatively.
| Table 30.5 Pros and cons of follicular unit extraction | |
| Pros | Cons |
|
|
Robotic FUE
Follicular unit extraction consists of two main steps: (1) separation of the follicular units from the surrounding skin, and (2) extraction (removal) of the follicular units from the scalp. Step one is a highly repetitive and labor intensive process that requires great precision. This step requires the centering of the punch over a follicular unit and the alignment of the dissecting instrument parallel to the follicles. Since this process must be repeated hundreds to thousands of times in a typical FUE hair transplant, it is subject to significant human variability and error.
 FIGURE 30.19 Robotic device used for follicular unit extraction.
FIGURE 30.19 Robotic device used for follicular unit extraction.A major advance in follicular unit extraction is the use of a robotically controlled extraction device that automates this crucial first step of FUE. ((ARTAS™ System manufactured by Restoration Robotics, Inc, Mountain View, CA.)) The robotic system increases the accuracy of graft harvesting, which in turn minimizes damage to hair follicles, potentially increasing graft survival.
The current robotic technology is based on the two-step method of extraction, namely, it uses a sharp punch to penetrate the skin and a dull rotating punch to separate the deeper part of the follicular unit from the surrounding tissue. The main improvement is a very precise, image-guided robotic arm that operates a dual-needle punch mechanism in order to increase the accuracy of extraction (Fig. 30.19).
Compared with manual systems, the robot is more versatile in its ability to harvest grafts from patients with different hair characteristics, patients from various ethnic backgrounds, and hair from different parts of the scalp. It is particularly useful in extracting grafts from the sides of the scalp, where the hair lies flatter on the skin.
Introduction of the Robotic system into a physician’s practice can present a formidable challenge. Besides the expense of the technology, the robot requires an operating room larger than those that exist in many doctors’ offices and requires that the room be dedicated to this purpose. ((Bernstein RM, Rassman WR. Pre-making recipient sites to increase graft survival in manual and robotic FUE procedures. Hair Transplant Forum Int 2012;22(4):128–30.)) In addition to special training required to operate the system, the FUE procedure itself should be modified, so that grafts are kept out of the body for as short a time as possible and kept in an environment that will ensure maximum growth. This can be accomplished by making recipient sites prior to the robotic harvesting and by using special biologic solutions to hold the grafts. ((Bernstein RM. Integrating Robotic FUE into a hair transplant practice. Hair Transplant Forum Int 2012;22(6):228–9.)), ((Bernstein RM. Robotic hair transplants. The Link 2013;5:6–7.))
Recipient sites
The way recipient sites are made determines the most critical aesthetic aspects of the transplant: the angle at which the new hair grows, its distribution, and its density. A thorough discussion of site creation is beyond the scope of this chapter, but the four basic elements will be reviewed briefly. These are recipient site size, hair direction, site density, and graft distribution, and they are covered in greater detail elsewhere.7
Recipient site sizes and orientation
Recipient sites should provide a snug fit for the grafts to prevent excessive coagulum formation between the grafts and the walls of the recipient site (which may inhibit proper oxygenation) and to hold it securely in place during post-op washing. However, it should not be so small that its insertion into the site is difficult or traumatic.1
Numerous instruments have been developed to create recipient sites. The main aspect to consider in choosing one is its diameter. ((Arnold J. Mini-blades and a mini-blade handle for hair transplantation. Am J Cosm Surg 1997;14:195–200.)) Although there are many viable options, the following are used by many practitioners:
- 21-gauge hypodermic needle for one-hair follicular unit grafts
- 20-gauge for thin two-hair follicular unit grafts
- 19-gauge needle for thick two-hair and all three- and four-hair follicular unit grafts
- 18-gauge for three-hair grafts in African ethnicities with very kinky hair.
These needles can be fitted on 1 mL or 3 mL syringes for ease of use, obviating expensive handles. Some physicians advocate smaller recipient sites, but this may require dividing four-hair follicular units – a technique that this author does not recommend.40 For very fine hair, or in special situations like transplanting eyebrows, a 22-gauge (or finer) needle can be used.
An alternative to using hypodermic needles, is to use a blade breaker that enables a straight blade to be custom cut into chisel-shaped cutting instruments of various sizes. It is this author’s opinion that hypodermic needles, which spread apart the skin as the wound is created, tend to cause less tissue injury than chisel-shaped blades, which cut through the skin.
Most instruments used to make sites create tiny slits as they cut through the skin and these slits can have either a coronal (horizontal) or sagittal (vertical) orientation. The advantages of coronal incisions are: a more natural appearance, since the original orientation of most follicular units seems to be coronal; a fuller look, since the hairs tend to fan out over the skin; and less tendency of the growing hair to elevate in the vertical plane. The advantages of a sagittal orientation are: greater visibility of sites; ease of graft placement; less trauma to existing hair; and less lateral (radial) splay of hair. Because coronal incisions provide better coverage and a slightly more natural look, they are currently used by the majority of hair transplant surgeons.
Hair direction
 FIGURE 30.20 Normal hair direction. (A) Top view and (B) side view.
FIGURE 30.20 Normal hair direction. (A) Top view and (B) side view.Hair should be placed into the scalp at the angle at which it originally grew, not in the direction that it is to be groomed. However, there are some exceptions. Whorls at the frontal hairline should not be mimicked with a hair transplant, nor should hair at the hairline be planted in a radial direction, even if some of the original hair grew that way. In general, hair anterior to the vertex transition point should point forward, with the angle becoming more acute as it reaches the anterior hairline – where it is essentially horizontal to the ground – regardless of the slope of the forehead. By planting the hair in the frontal hairline in a forward direction (rather than radially), the transplanted hair will act more as a unit and hold together when it is groomed (Fig. 30.20).7 At the temples, the forward-pointing hair makes an abrupt transition to a downward direction. Hair at the temples should be transplanted at a very acute angle that is lying as close to the surface of the skin as possible.
Recipient site density
The average non-balding scalp has 70–95 follicular units per cm2. Approximately 50% may be lost before there is any noticeable thinning. It would be wasteful for more than 50% to be replaced, especially since hair transplants are always performed in the face of diminished total hair volume. We generally recommend transplanting up to 25–30 follicular units/cm2 into the frontal area of a balding scalp in the first session. If the larger three-hair and four-hair units are placed in select areas, more than 25% of the initial density can be achieved in one pass. The ideal transplant density can be achieved in many patients with two procedures.7
Some physicians advocate a “one-pass” procedure to achieve the final density.40 Although this may be appropriate for some patients, the increased incidence of graft popping and desiccation, insertion injury, and possible vascular compromise may lead to poor growth. For very bald patients, very dense packing does not allow coverage of an entire bald area.
Transplants of more than 3000–3500 follicular unit grafts may necessitate that the grafts be out of the body so long that their survival may be diminished. In addition, the large donor strip required to harvest graft numbers of this magnitude increases the incidence of a widened donor scar. Furthermore, for procedures of greater than 3000–3500 to be practical, if not already bald, the recipient area should be shaved – a situation impractical for many working patients.
It is this author’s view that transplanting the entire region to be covered in the first session and then increasing density in a subsequent session is a better goal for the majority of patients than to transplant maximum density in one section and then do another part of the scalp at a later date.
Graft distribution
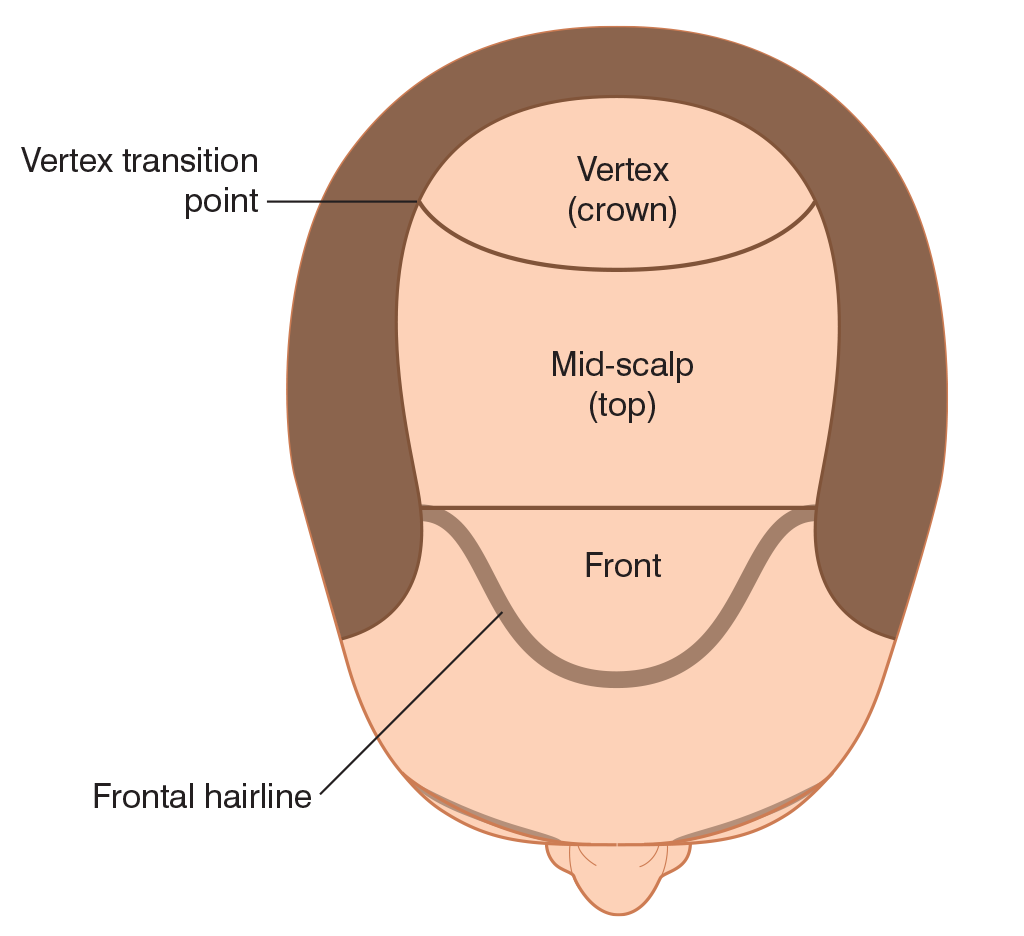 FIGURE 30.21 Regions of the scalp.
FIGURE 30.21 Regions of the scalp.For simplicity, the area of the scalp subject to androgenetic change can be divided into three regions: the frontal region that includes the frontal hairline; the top or mid-scalp; and the vertex or crown. The vertex transition point separates the top of the scalp from the crown (Fig. 30.21).38 As most social interaction takes place face-to-face, and people generally view themselves from the front, the overall impact of the transplant is essentially defined by the position of the frontal hairline and density of the frontal region of the scalp.
For most patients who are moderately or extensively bald, or for those who are destined to be, the limitations of the donor supply make restoring the entire bald scalp to ideal density impossible. Consequently, creating the greatest density in the frontal forelock area of the scalp produces the best cosmetic result. This “forward weighting” can be accomplished by creating recipient sites closer together, placing larger follicular units (i.e., those with three and four hairs) in those sites, or by a combination of both.
 FIGURE 30.22 Schematic of “forward weighting,” achieved by close placement of recipient sites (shaded dark grey), and central density, accomplished by placing larger follicular units in the forelock area (dark brown oval).
FIGURE 30.22 Schematic of “forward weighting,” achieved by close placement of recipient sites (shaded dark grey), and central density, accomplished by placing larger follicular units in the forelock area (dark brown oval).In general, recipient site density should be the highest in the front part of the scalp and gradually tapered toward the crown. In contrast, the largest follicular units should be placed in the forelock region of the scalp. This overlapping distribution of sites and follicular unit grafts may be visualized in Figure 30.22. It is explained in greater detail elsewhere.7 The pattern serves two functions: (1) it creates a natural central-forelock density without the need for spacing sites closer towards the mid-scalp, where the blood supply can most easily be compromised; and (2) it insures the most natural look by placing the larger follicular units in a forward-central position, but away from the frontal hairline. Density in the forelock area brings about a “patterned look” and avoids the unnatural, diffuse thinning that results when small grafts are evenly distributed over the scalp.
The majority of patients have enough donor hair to provide at least light coverage extending to the vertex transition point. This is a natural stopping point because even if the crown continues to enlarge. Transplants performed to this point will continue to look natural without additional surgery. Transplants should be extended past the vertex transition point into the crown only when there is adequate hair to create a swirl and follow the hair loss if the balding progresses. The indications for transplanting the crown are discussed in detail elsewhere.6
 FIGURE 30.23 Left shows schematic view of left-to-right “side weighting.” Right shows extreme side weighting employed when the donor supply is severly diminished.
FIGURE 30.23 Left shows schematic view of left-to-right “side weighting.” Right shows extreme side weighting employed when the donor supply is severly diminished.In the first transplant session, grafts should generally be transplanted in a symmetric distribution. However, once the first transplant has had a chance to grow and the patient is willing to commit to styling his/her hair in a specific pattern, “side weighting” may be considered. This is accomplished either by placing a greater proportion of the grafts on the parted side of the scalp, using larger follicular units on that side, or doing both. It results in increased fullness when one’s hair is combed to the side. In situations where there is a great imbalance in the supply/demand ratio, such as after scalp reductions or old plug procedures, a more exaggerated form of “side weighting” should be considered. In this case, the follicular unit grafts are concentrated on the front and parted side of the scalp, but widely scattered on the top and back (Fig. 30.23).7
Graft placement should be carried out in a front-to-back sequence, beginning at the frontal hairline starting with one-hair follicular units. In a typical transplant, approximately 250–300 one-hair units are used for the leading edge of the frontal hairline, immediately followed by two-hair grafts. Three- and four-hair units are concentrated in the forelock area, while toward the back and sides the pattern is reversed so that the three- and four-hair units are always central to the one- and two-hair follicular unit grafts.
 FIGURE 30.24 Staff using loop magnification and curved jeweler’s forceps to place follicular unit grafts into pre-made recipient sites.
FIGURE 30.24 Staff using loop magnification and curved jeweler’s forceps to place follicular unit grafts into pre-made recipient sites.Approximately 20–30 grafts are transferred at one time from the dish to the index finger and held in a droplet of holding solution. The grafts are inserted using curved jeweler’s forceps (Fig. 30.24). A small Band-Aid should be worn on the finger under the area the grafts will sit, to decrease the incidence of a stick injury from the forceps and to minimize the transfer of heat from the hand. Each graft should be carefully grasped by the fat at its bottom or at its edge, not at the germinative center. In the two-handed technique, gauze – held between the free fingers of the hand holding the grafts – is used to keep the partially inserted grafts in place as the forceps are re-positioned higher along the length of the graft to facilitate the last phase of insertion. This is a particular useful technique when there is “popping” (the tendency of grafts to lift up as they are being placed.)
Most physicians create the majority of recipient sites prior to graft placement. This allows the doctor to concentrate on the angle and distribution of sites without having to worry about actual graft placement. It also allows the team to easily clean the recipient area without having to be concerned about dislodging grafts. In addition, it gives time for some coagulation to occur and for the sites to become more “sticky” as this holds the grafts in place and reduces the incidence of popping.
In an alternative method, termed “stick and place,” the grafts are inserted at the same time that the recipient sites are made.40 In this technique, the needle that is used to make the recipient hole can serve as a “shoehorn” to help guide the graft into the site. The advantage of this method is that it eliminates the possibility that sites may be left unfilled or that two grafts may be placed into one site (piggy-backing). It also moves the surgery along faster; since other members of staff do not need to wait for the surgeon to make the sites and they do not need to search for the sites that are empty.
On the other hand, there is more bleeding when sites are made concomitantly with graft placement (decreasing visibility) and there is an increased risk of the grafts popping and then drying out on the surface of the scalp. In addition, the staff must make judgments regarding the angling and distribution of the sites they are creating at a time when they must also be focusing on the technical aspects of graft insertion. This leaves many of the aesthetic decisions in the hands of the technicians (who are consumed with the job of stick and place), rather than with the physician who should be taking a more global view of the transplant design.
Patient disposition
After graft insertion is complete, the scalp is cleaned with distilled water, bacitracin zinc ointment is applied to the stapled area, and a headband-type pressure dressing is placed over the donor area. The transplanted area is covered with a surgeon’s cap. Prior to leaving, patients are given verbal and printed postoperative instructions, and medications for sleep and pain (Table 30.6). Patients leave the office wearing a bandanna covering the cap and the headband. It is worth stressing that patients given sedatives or pain medication should not drive after surgery.
Table 30.6 Common medications in hair transplant
| Class | Medication | Use |
| Topical antibacterials | Hibiclens | Preoperative |
| Systemic antibiotics | Dicloxacillin,cephalosporin | Intraoperative prophylaxis (a) |
| Sedatives/hypnotics | Diazepam, aprazolam ((Weber P, Weber R, Dzubow L. Sedation for dermatologic surgery. J Am Acad Dermatol 1989;20:815–26.)) | Intraoperative sedation |
| Corticosteroids | Betamethasone | Postoperative swelling |
| Topical antibiotics | Bacitracin ointment | Postoperative care, donor area |
| Opiates | Hydrocodone | Postoperative pain, prn |
| Anti-hiccup agents | Chlorpromazine | Hiccups, prn |
| Analgesics | Nitrous oxide62 | During injections prn (b) |
| Wound healing promoters | Copper-peptide | Postoperative healing (b) |
| Hair-growth promotors | Finasteride, minoxidil | Pre- and postoperative (a) |
(a) See text.
(b) Not used in author’s practice.
OPTIMIZING OUTCOMES
Transition zones
A soft natural hairline is the hallmark of the ideal hair transplant. However, careful observation of a normal frontal hairline reveals that there is no actual “line,” but rather an irregular, slightly asymmetric “transition zone” or gradation of follicular units of increasing size and density. This is what should be replicated in the transplant (Fig. 30.25). Transition zones are not limited to the frontal hairline; they must be recreated wherever the edge of the transplanted area is visible. In Figure 30.25C, one can see that transition zones have been created at the frontal hairline, sides, and vertex transition point leading to the crown.
Although there are many ways of creating a transition zone at the frontal hairline, the placement of approximately 250–300 one-hair follicular units will usually achieve the appropriate softness. An irregular hairline can be achieved with these one-hair grafts by creating triangles of varying size and shape. Immediately behind this, one should place two-, three-, and eventually four-hair units. The larger units should be more centrally located (Fig. 30.25B). With finer hair, larger units may be placed slightly closer to the hairline.


 FIGURE 30.25 (A) Before. (B) Preoperative markings of the planned procedure. (C) First session just before placement, showing the distribution of 2355 recipient sites. (D) At 1 year, after two sessions totalling 4295 follicular unit grafts.
FIGURE 30.25 (A) Before. (B) Preoperative markings of the planned procedure. (C) First session just before placement, showing the distribution of 2355 recipient sites. (D) At 1 year, after two sessions totalling 4295 follicular unit grafts.Visibility
Pre-making all the recipient sites before graft insertion helps to control bleeding and limits the need for epinephrine (Fig. 30.25C). It initiates the “extrinsic pathway” so that coagulation can begin before the grafts are introduced and allows for easy cleaning of the recipient area of any blood or coagulum, without the risk of dislodging grafts. It also makes it possible to place sites close together without a concern for grafts in adjacent sites popping. Most importantly, it provides maximum visibility in the placing phase of the procedure.51
If the initial sites are scattered, they tend to cause the cutaneous vasculature of the scalp to “clamp down,” increasing visibility for subsequent passes and allowing the skipped areas to be filled in. When pre-making sites, the logistics of matching the number of sites to the number of grafts can be solved by making “projections” of the anticipated number of grafts, while the dissection phase is still in progress.
Recipient site influences

 FIGURE 30.26 Norwood class VI, with very fine, blond hair, and a donor density of 2.5 hairs/mm2. (A) Before and (B) 1 year after two sessions of 2678 and 1836 follicular unit grafts.
FIGURE 30.26 Norwood class VI, with very fine, blond hair, and a donor density of 2.5 hairs/mm2. (A) Before and (B) 1 year after two sessions of 2678 and 1836 follicular unit grafts.
 FIGURE 30.27 Early Norwood VI. (A) Before procedure. (B) 1 year after one session of 2520 follicular unit grafts.
FIGURE 30.27 Early Norwood VI. (A) Before procedure. (B) 1 year after one session of 2520 follicular unit grafts.Some hair characteristics, such as wave, are governed by the recipient site. When individual follicular units are placed into very small recipient wounds, there is little fibrotic reaction to the grafts and the recipient area can exert its influences on hair growth. In Figure 30.26, note the delicate wave produced by the recipient scalp. It sometimes takes a year or more for the hair character to return to normal.
Hair direction
When the hair is planted at the frontal hairline in its natural forward-pointing direction, combing it back causes it to bow, giving it the appearance of fullness. A common mistake is to point the hair in the direction that the patient plans to comb their hair; this makes the hair lie flat and bodiless when groomed. On the other hand, as the frontal hairline approaches the temples, the hair direction should be abruptly changed, so that it is angled downward and slightly backward, lying very flat on the scalp. Perfect integration of the frontal hairline with hair on the sides and temples is essential if the transplant is to look completely natural (Fig. 30.27).
Hairline placement
If it is to look natural throughout the patient’s life, the transplanted hairline must simulate that of a mature adult. In the adolescent male, the frontal hairline touches the top crease of the forehead when the eyebrows are raised (that is, the hair begins at the upper border of the frontalis muscle). In a mature adult, the mid-portion of the frontal hairline rests approximately one finger-breadth (1.5–2 cm) above the upper brow crease. Although the degree of temple recession can vary dramatically in the normal adult hairline, the mid-part of the frontal hairline is crucial in framing the face.1,7 Significant bitemporal recession was built into the restoration of the patient in Figure 30.28, yet the forward-placed mid-portion sets his facial features in the correct proportions.

 FIGURE 30.28 Early Norwood class V. (A) Before procedure. (B) 1 year following two sessions of 2105 and 1665 follicular unit grafts.
FIGURE 30.28 Early Norwood class V. (A) Before procedure. (B) 1 year following two sessions of 2105 and 1665 follicular unit grafts.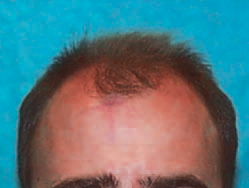
 FIGURE 30.29 Norwood class V. (A) Before procedure. (B) Integrating hair with a persistent frontal forelock (1 year following two sessions of 2133 and 1171 follicular unit grafts).
FIGURE 30.29 Norwood class V. (A) Before procedure. (B) Integrating hair with a persistent frontal forelock (1 year following two sessions of 2133 and 1171 follicular unit grafts).
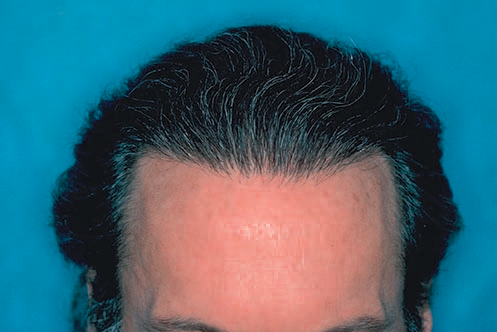 FIGURE 30.30 A 46-year old with early Norwood class IVA and wavy medium-coarse hair. (A) Before transplant. (B) After two sessions of 2085 and 1438 follicular unit grafts.
FIGURE 30.30 A 46-year old with early Norwood class IVA and wavy medium-coarse hair. (A) Before transplant. (B) After two sessions of 2085 and 1438 follicular unit grafts.Forelock integration
A persistent frontal forelock poses an interesting dilemma. When hair is transplanted around the forelock, the patient may be left with a gap in his frontal hairline if the forelock eventually disappears. On the other hand, it is wasteful to add hair to a forelock with adequate density that may persist for years. The solution is to check for miniaturization with a densitometer. The patient in Figure 30.29 had miniaturization in his forelock greater than 50%, suggesting that it would not be stable over time. Two sessions were used to integrate transplanted hair into the resident terminal hair of the forelock, producing a density that will be permanent.
Optimizing density
One of the advantages of FUT over micrografting is the ability to sort follicular units and place the larger three- and four-hair units in the forelock area, creating a density significantly greater than if the grafts were evenly distributed. This is possible because the compactness of the follicular unit allows four-hair grafts to be placed into very small sites.5,7 In certain instances, follicular units may contain more than four hairs, and adjacent follicular units are occasionally so close together that they can fit into a small recipient site. ((Seager D. Dense hair transplantation from sparse donor area – introducing the “follicular family unit.” Hair Transplant Forum Int
1998;8:21–3.)), ((Tykocinski A. A one-year study of using exclusively “follicular grouping grafts” in specific areas to increase hair density and volume during FUT. Hair Transplant Forum Int 2003;13:366,369–700.)) However, as long as these “grouped grafts” are placed in a central location and the recipient wounds are kept small, this technique can add density without risking an unnatural look. It is important to keep in mind that ultimate density depends on the absolute number of hairs moved rather than the size of the grafts. ((Limmer BL. The density issue in hair transplantation. Dermatol Surg 1997;23:747–50.)) Using grafts larger than follicular units always risks that the transplant may look unnatural. The patient in Figure 30.30 achieved a very dramatic frontal density in two sessions using the technique of follicular unit sorting.
Creating camouflage
A significant part of many practices involves repairing transplants performed with older techniques. FUT is ideal for this because of the small size of follicular units and high hair count. ((Bernstein RM, Rassman WR, Rashid N, et al. The art of repair in surgical hair restoration – part I: basic repair strategies. Dermatol Surg 2002;28:783–94.)), ((Bernstein RM, Rassman WR, Rashid N, et al. The art of repair in surgical hair restoration – part II: the tactics of repair. Dermatol Surg 2002;28:873–93.)) Often fixing a “pluggy” look requires graft excision with re-implantation, before the actual transplant. The patient in Figure 30.31 had a row of plugs set far enough back from his frontal hairline that camouflage with follicular units alone was sufficient for the repair. In general, however, it is best to excise the larger grafts, divide them into individual follicular units and redistribute them in a more natural location and direction, than to merely camouflage them.

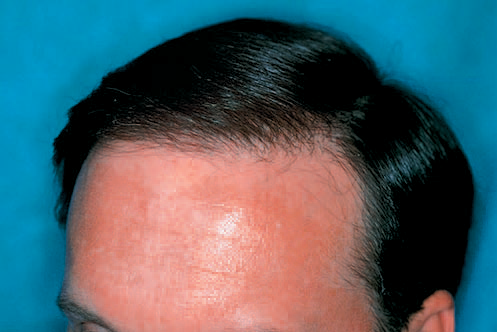 FIGURE 30.31 (A) Before transplant, showing large grafts forming a literal wall of plugs. (B) Camouflage from one session of 1818 follicular grafts.
FIGURE 30.31 (A) Before transplant, showing large grafts forming a literal wall of plugs. (B) Camouflage from one session of 1818 follicular grafts.Eyebrow transplantation
Eyebrows are perhaps more important to one’s appearance than scalp hair, as eyebrows are in a more central position on the face and serve to frame the eyes. Unlike the loss of scalp hair, the loss of one’s eyebrows is not viewed as a natural process and is, therefore, never cosmetically acceptable. Because eyebrows have their own unique attributes, eyebrow transplants require techniques different from hair transplants on the scalp: (1) eyebrow hair grows as individual strands, therefore multi-hair follicular units should be divided into 1-hair micrografts before planting; (2) eyebrow hair must be planted at a very acute angle so that the hair grows as flat as possible to the surface of the skin; (3) subtle angle changes are required to recreate the fanlike splay of hair at the medial aspect of the brow; (4) the hair must be planted in a criss-cross growth pattern that causes the hairs in the middle of the eyebrows to converge and form a subtle natural elevation; and (5) since the hair cycle of scalp hair (3–7 years) is longer that eyebrow hair (4 months), the eyebrow hair (after a transplant from the scalp) must be periodically trimmed. ((Epstein JS. Eyebrow transplantation. Hair Transplant Forum Int 2006;16:121–3.))
Eyelash transplants
The donor hair used in an eyelash restoration must match, as closely as possible, the original eyelash. Hair suited for this purpose includes the fine short hairs at the nape of the neck, hair from behind the ears or the eyebrows. The incision made into the lid margin should be made with a curved instrument and the hair should be rotated to follow the curvature of the original lashes. ((Jazayeri K. Eyelash transplantation using eyebrow-derived single-hair grafts: a case report. Hair Transplant Forum Int 2008;18: 134–5.)) In a very elegant procedure, scalp hairs are threaded onto a crescent needle and then introduced into the eyelid through an incision made on the inside of the lid margin. The hairs are then cut to the appropriate length. Regardless of the technique, trichiasis (eyelash misdirection) is a potential significant complication that can lead to impaired vision. Eyelash transplants should be performed by only the most experienced surgeons. ((Caputy GG, Flowers RS. The “pluck and sew” technique of individual hair follicle placement. Plast Reconstr Surg 1994;93: 615–20.))
Long hair transplants
Transplanting long hairs, instead of the traditional 1–2 mm clipped hairs, allows the patient to temporarily see the actual results immediately upon completing the procedure. In this technique, the donor hair is left long (up to 25 cm) throughout the harvesting, dissecting, and placing steps. As with traditional hair transplants, the hair is shed about 4 weeks after surgery. Although the procedure gives the patient an early view of the results and eliminates the problems of piggy-backing and buried grafts, it is technically more laborious and requires more meticulous postoperative care. A significant risk is that the longer hair grafts can more easily be dislodged from the scalp. ((Bouhanna P. Long hair grafts: 20 years of experience. Hair Transplant Forum Int 2007;17:127–8.)), ((Pitchon M. Preview. Long-hair follicular unit transplantation: an immediate temporary vision of the best possible final result. Hair
Transplant Forum Int 2006;16:114, 118–119.))
Trichophytic donor closure
A trichophytic closure is a way of improving the appearance of the linear donor scar from strip harvesting. A trichophytic closure involves trimming the upper or lower edge of the incision and then closing the wound in such a way that the hairs near the edge can grow toward or through the scar. This has the potential to produce a linear donor scar that is virtually undetectable. ((Marzola M. Trichophytic closure of the donor area. Hair Transplant Forum Int 2005;15:113, 116–117.))
Automation
Robotic devices are now used in FUE procedures to isolate follicular units from the surrounding tissue prior to their removal from the donor area. However, graft harvesting is only the first of the three major steps of a hair transplant. Within the next few years, robotic technology is expected to extend to recipient site creation. Before the end of the decade we should be witnessing the fully automated placement of grafts.70, ((Rassman WR, Bernstein RM. The automation of hair transplantation: past, present, and future. In: Harahap M, editor. Innovative techniques in skin surgery. New York: Marcel Dekker; 2002. p. 489–502.)) Although robotic technology virtually eliminates the risks of human variability and error, the full automation of a procedure that is labor intensive, organizationally complex and, most importantly, dependent on the aesthetic judgment of the surgeon remains a formidable challenge.
Hair cloning
The ability to clone human follicles will revolutionize surgical hair restoration as this will provide an unlimited supply of donor hair. Cloning will potentially allow older patients to enjoy a full head of hair and younger patients to have their adolescent density and hairline restored. One area of research is follicular cell implantation (FCI). FCI is based on the ability of the dermal papilla cells (fibroblasts), found at the bottom of hair follicles, to interact with the keratinocytes and stimulate new hairs to form. In this technique, dermal papilla cells can potentially be grown and multiplied in culture, so that a very small number can theoretically produce enough follicles to cover an entire bald scalp. ((Teumer J. Strategies for follicular cell implantation. Hair Transplant Forum Int 2008;18:97–8.)) Another method is to co-culture epidermal and dermal cells in-vitro to produce a population of “proto-hairs” that could be implanted into the scalp. ((Qiao J, Turetsky A, Kemp P, et al. Hair morphogenesis in vitro: formation of hair structures suitable for implantation. Regen Med 2008;3(5):683–92.)), ((Cooley JE. Cell-based treatments for hair loss: research update on “hair cloning.” Hair Transplant Forum Int 2013;23(2):47–9.))
There are a number of obstacles that still need to be overcome before cloning can be a viable option for patients. First, cells seem to lose their inductive properties when they are cultured over multiple generations; second, the production of cosmetically useful hair – which depends on the interaction between the various cell types – is not yet possible; and third, research in its preclinical phases can take years before it receives approval by the US Food and Drug Administration (FDA).
POSTOPERATIVE CARE
Patients are called at home the day after the procedure. Those with a stapled closure are seen in our office 10 days postoperatively to have alternating staples removed. They return again 10–14 days later to have the remainder of the staples removed. Patients in whom we have used sutures return for only one visit 7–10 days postoperatively. Patients who have had follicular unit extraction are seen at 1- and 2-weeks postoperatively.
At 1 year postoperatively, all patients return to the office so that we can assess growth, take photographs, and discuss the possibility of additional surgery. Those with questions or concerns are seen as frequently as needed. Box 30.6 summarizes typical postoperative instructions for a typical follicular unit transplant procedure. In FUE, the patient is instructed to keep the donor area covered with a thick layer of antibiotic ointment during the day and covered with ointment and Telfa gauze at night.
| Box 30.6 Summary of instructions following follicular unit hair transplantation |
| Care of transplanted area Shampoo every 3–4 h the day following surgery and then twice daily until the 1-week postoperative visit. Be especially careful when cleaning the transplanted site in the first few days so that the grafts will not be dislodged. However, a thorough but gentle rinsing of the recipient area will minimize crusting and make the transplant less noticeable. When shampooing and rinsing the transplanted area, be gentle for the first 2 weeks following surgery. Do not rub, pick, or scratch, as this may dislodge grafts. Care of sutured/stapled area in FUT Gently wash the sutured area with your fingertips using a copper-peptide-containing shampoo. Shower water may hit the sutured area in the back of the scalp directly. A hair dryer may be used when set on warm, but not hot. Do not use any scalp- or hair-coloring agents for at least 2 weeks after the surgery. Care of the donor area in FUE Gently wash the donor area with your fingertips using a copper-peptide-containing shampoo. Shower water may hit the donor area in the back of the scalp directly. After washing, apply a thick layer of antibiotic ointment to the area. Cover the area with Telfa gauze, held in place with a head-band, while sleeping. Postoperative medications After surgery, sleeping medication and medication for pain may be given. Itching There may be some itching either in the transplanted area or sutured area following surgery. Hydrocortisone ointment may be applied locally to the areas that itch as needed, up to four times a day. Swelling Oral corticosteroids may be given during and after the procedure to decrease swelling following surgery. If there is significant swelling, it usually occurs around the 2nd to 5th postoperative day and should not be cause for concern. It resolves by itself after a few days and does not require any special treatment. Cool, wet dressings may be placed over the swollen area, but make sure they do not touch the grafts. Bleeding Before leaving the office, all bleeding will be controlled. Rarely, some bleeding may occur after the procedure. If this happens, apply firm continuous pressure on the area. If it is impossible to contact the office, go to the nearest emergency room and show these instructions to the physician on duty. Exercise, alcohol, smoking, and other restrictions Avoid direct trauma to the head for 10 days after the procedure, and abstain from sexual intercourse, alcohol use, and smoking for 3 days after the procedure. After 10 days, normal daily activities may be resumed. Be careful not to stretch the donor area until instructed by the physician (FUT only). Sun Avoid unprotected exposure to strong sunlight for 3 months. Wear a hat when going outside or use a strong sunscreen with a sun protection factor of 30+. Infection Redness, swelling, and slight tenderness are to be expected for the first few days after a procedure. Persistent swelling, pain, or tenderness in the sutured area may be a sign of infection. Fever and/or chills are indications of infection as well. There also may be a discharge or pus in the suture line. If any of these conditions should occur, please contact the office. Numbness Scalp numbness, tingling, or similar sensations may occur temporarily. This generally disappears within a few weeks to months, as nerve endings regrow. Hair-loss medications Minoxidil 5%: If the plan is to continue this medication, resume it 1–2 weeks after the procedure. Finasteride 1 mg: This medication should not be discontinued for the surgery. |
Table 30.7 Pitfalls of follicular unit hair transplantation and their management
| Problem | Cause | Prevention | Treatment |
|---|---|---|---|
| Syncope | Anxiety, vasovagal reaction | Pre-medicate with diazepam or atropine, administer local anesthesia with patient lying flat |
Lie patient supine and elevate legs |
| Anxiety, palpitations | β1- and α-adrenergic effects of epinephrine | Administer epinephrine slowly, pre-medicate with diazepam | Patience (the half-life of epinephrine is short); lidocaine for arrhythmias |
| Hypertension, bradycardia | Epinephrine interaction with β-blocker | Use selective β1-blockers | α-Adrenergic blockers; treat as cardiac emergency |
| Agitation, confusion, perioral numbness | Lidocaine toxicity | Use more dilute anesthetic mixture, use ring blocks rather than local infiltration | Intravenous fluids, oxygen, ventilate, diazepam; treat as cardiac emergency |
| Pruritus, urticaria, angioedema | Allergic reaction or anaphylaxis to drugs or food | Careful history | Oxygen, epinephrine subcutaneously |
| Excessive bleeding | Elevated blood pressure, non-steroidal anti-inflammatory agents |
Discontinue antiplatelet agents before surgery, continue antihypertensive medications on day of surgery, pulse oximetry | Operate with patient seated; with elevated blood pressure, consider antihypertensives |
| Postoperative edema | Surgical trauma, buffered anesthetic | Keep recipient sites small, preoperative and postoperative systemic corticosteroids | Head elevation, additional postoperative corticosteroids |
| Sterile folliculitis | Growing hair trapped by epidermal overgrowth | Keep grafts slightly elevated | Resolves spontaneously; incision and drainage if persistent |
| Cyst formation at graft site | Foreign body reaction to imbedded graft | Avoid piggy-backing one graft over another in the same site | Incision and drainage, topical and/or systemic antibiotics |
| Bacterial folliculitis | Picking, scratching, poor hygiene, secondary to infected sterile folliculitis or cyst | Frequent postoperative showering, topical corticosteroids for pruritus | Topical and systemic antibiotics |
| Infected donor wound | Suture bites too large or tight; with postoperative edema, causes vascular compromise | Harvest a narrower donor strip under less tension, change suturing technique | Systemic antibiotics; if severe, leave portion of donor area to heal by second intention, watch for MSRA ((True R. Hair restoration in the age of MRSA. Hair Transplant Forum Int 2008;18:121, 129, 130.)) |
| Widened scar in donor area | Donor strip too wide or placed too low, patient tendency to heal with stretched scar | Careful history, conservative donor strip located at level of occipital protuberance | Excision ± (can worsen scar), use FUE instead |
| Raised donor scar | History of keloids, racial susceptibility, incision placed too low | Careful history, patient selection, test biopsy | Intralesional corticosteroids |
| Persistent numbness or paresthesias in the back of the scalp | Transection of branches of the occipital nerves | Limit donor depth to deep subcutaneous layer, use tumescent anesthesia | Subsides spontaneously if the cut nerve branches were small |
| Hiccups | Possible injury to C2–C4 indirectly stimulating the vagus or diaphragmatic nerves | Limit donor depth to deep subcutaneous layer, use tumescent anesthesia | Chlorpromazine |
| Hair texture changes | Trauma to grafts, sebaceous glands removed in dissection | Careful atraumatic dissection and placing, avoid trimming follicular units too close | Usually resolves spontaneously |
| Skin texture changes | Recipient wounds too large | Make wounds no larger than the equivalent size of a 19-gauge needle at the frontal hairline | Add coverage with one- and two-hair follicular units at the frontal hairline using very small sites |
| Hair loss in recipient area related to the procedure | Telogen effluvium ((Headington JE. Telogen effluvium: new concepts and review. Arch Dermatol 1993;129:356–63.)) | Possibly effective: small surgical wounds, limit epinephrine, pre-treat with finasteride | Terminal non-miniaturized hair should return spontaneously, finasteride |
| Hair loss in donor area | Strangulation of follicles by sutures, transection of follicles during harvest | Narrower donor strip, meticulous suturing techniques | Follicular unit transplantation, follicular unit extraction, local excision |
| Failure of transplanted hair to grow | Desiccation; ((Gandelman M, Mota AL, Abrahamsohn PA, et al. Light and electron microscopic analysis of controlled injury to follicular unit grafts. Dermatol Surg 2000;26:31.)) crush injury; ((Greco J. The H-factor in micrografting procedures. Hair Transplant Forum Int 1996;6:8–9.)) transection; ((Cooley J, Vogel J. Loss of the dermal papilla during graft dissection and placement: another cause of X-factor? Hair Transplant Forum Int 1997;7:20–1.)), ((Limmer BL, Razmi R, Davis T, et al. Relating hair growth theory and experimental evidence to practical hair transplantation. Am J Cosmet Surg 1994;11:305–10.)), ((Bernstein RM. Blind graft production: value at what cost? Hair Transplant Forum Int 1998;8:28–9.)), ((Bernstein RM, Rassman WR. Follicular unit graft yield using three different techniques. Hair Transplant Forum Int 2001;11:1, 11–13.)) grafts out of the body too long53 | Keep grafts well hydrated in holding solution, careful microscopic dissection and handling | If cause can identified and corrected, then re-transplant |
MRSA, methicillin-resistant Staphylococcus aureus.
PITFALLS AND THEIR MANAGEMENT
A number of problems arising in hair transplant surgery have been well described in the literature. These are summarized in Table 30.7, along with their most likely cause, although some etiologies may be multifactorial. The table also summarizes and methods for preventing and managing them should they occur.11, ((Meza DP. Complications in hair restoration surgery. Hair Transplant Forum Int 2000;10:145.)), ((Fader DJ, Johnson TM. Medical issues and emergencies in the dermatology office. J Am Acad Dermatol 1997;36:1–16.))
Although FUHT eliminates many of the shortcomings of the older techniques, such as a “pluggy” look, a “moth eaten” donor area, or midline scalp reduction scars, poor aesthetic judgment and techniques that compromise graft growth can still lead to problems. Perhaps because FUHT requires large numbers of grafts (using a significant portion of the donor area at one time), because so many staff members are involved in the process, and because some of the problems of small-graft procedures are very difficult to correct, improperly performed FUHT can pose a greater risk to patients than traditional grafting. The problem is compounded by the fact that many physicians perceive FUT, and especially FUE, as a safe, risk-free procedure and describe it as such to patients.
The remainder of this section will focus on some of the most common mistakes made by FUHT practitioners, particularly in the areas of planning, transplant design, and handling large numbers of small grafts. These problems and how they may be avoided are summarized in Box 30.7.
| Box 30.7 Thirteen common pitfalls in follicular unit hair transplantation |
|
Operating on patients that are too young or before medical therapy
Patents in their early 20s have their flat adolescent hairline and original density fresh in their memory. A transplant designed with enough frontal and temporal recession to look good for one’s entire life will rarely satisfy a younger patient. Creating a density that suits a younger person early hair loss will not leave enough hair in reserve when their balding progresses. In addition, at this age, the extent of future balding is very difficult to anticipate. For these reasons, surgery should rarely be considered in patients with androgenetic alopecia who are younger than 25 years of age.
Often, doctors begin medical therapy and schedule surgery at the same time. However, if there is a possibility that using medication, such as finasteride and minoxidil, can make transplantation unnecessary, then the medication should be used for at least a year before any decision on surgery is reached. Medication should be the first line of therapy for all younger patients with androgenetic alopecia, regardless of the degree of their hair loss.
Failing to identify low donor density before surgery
Earlier in this chapter, the importance of assessing patient’s donor supply with densitometry was stressed. A low donor density, generally <1.5 hairs/mm2, usually indicates that donor supply is insufficient to create adequate density or coverage and is a relative contraindication to surgery. An exception might be an older person with very conservative goals. High miniaturization in the donor area, particularly in a person under the age of 30, is indicative of DUPA and is a contraindication to surgery.6
Transplanting young patients with low donor density will not only insure a poor cosmetic result but also will risk a visible donor scar. FUE, which is sometimes considered in such cases, is also not appropriate since, like FUT, it success depends on sufficient donor hair. Because the contrast between bald and non-balding scalp in patients with low donor density is already low, their best option is often wearing their hair short to further decrease the contrast. Close-cropped hair is a much better option for these patients than surgery.
Failing to identify a tight scalp
Assessing scalp laxity is an under-appreciated aspect of the patient evaluation, probably because it is difficult to quantify. A tight scalp severely limits the total amount of hair harvestable with an FUT procedure and can constitute a contraindication to this technique, except when patients have extremely conservative goals or are expected to experience only limited balding. The constraints that poor scalp laxity impose generally manifest themselves after the first transplant session. Though laxity should be judged in the preoperative evaluation, the intraoperative assessment made while suturing, is quite accurate in predicting future difficulties. To this end, every operative report should include a record of the ease of closure and intraoperative suture tension.6
A tight scalp can be loosened with repeated massage. The patient should begin by placing the palm of the hands on the back/sides of the head with the fingers pointing upwards and thumbs pointing towards each other. The scalp is then moved up and down as vigorous as possible using the palms and thumbs, with the fingers resting stationary on the top of the scalp. The exercise should be performed for a total of 15 min every evening at bed time. One should try to begin the exercise at least 8 weeks prior to surgery and continue the exercise up to the day of the procedure. ((Mohebi P, Pak J, Rassman W. How to assess scalp laxity. Hair Transplant Forum Int 2008;18:161, 167.))
Harvesting a donor strip that is too wide
In large FUT sessions, it can be tempting to take a slightly wider donor strip in order to conserve on length. A strip that is 25 cm by 1 cm, for instance, can be shortened by 6 cm if widened by just 3 mm and yield the same amount of hair. However, a wide strip puts unnecessary tension on the donor closure and is probably the most common iatrogenic cause of widened scars. If larger sessions are appropriate, and the scalp lacks adequate mobility, the surgeon should consider a longer incision rather than a wider one.
If a wide donor strip has been identified as the likely cause of a stretched scar, it is advisable to wait at least 8–12 months, to give the scar a chance to mature and regain some of its original laxity. When the next excision is made, the strip should measure at least 3–6 mm narrower than the previous one. Attempts to remove the entire width or length of the old scar invariably lead to a recurrence, or worsening, of the old scar. To facilitate healing, the new excision should extend to the patient’s hair-bearing edge, and be significantly shorter than the old incision.
Unfortunately, attempts to re-excise scars commonly result in either no improvement or an even wider defect. For this reason, we have been using FUE to place hair directly into the scar as a primary treatment. It is has been our experience, however, that transplanted hair will not grow in a thickened scar; therefore any hypertrophic scars should be thinned with intralesional corticosteroids prior to implantation and then a small test area should be transplanted first, to see if the hair will grow and to make sure that the scar does not thicken from the grafting.80
It has been recommended to use small punches (0.6–0.7 mm) to create the recipient sites when transplanting into thickened scars. The rational is that it will de-bulk the scar and create space for the grafts, thereby limiting compression injury in the crucial first 3 days after surgery, when the inflammatory/edematous response is at a peak. ((Martinick J. Hair transplantation into scars. Hair Transplant Forum Int 2013;23(2):58–9.))
Placing the donor incision too low or too high
The ideal position for the donor incision is in the midportion of the permanent zone. In most people, this lies at the level of the external occipital protuberance and the superior nuchal line. The muscles of the neck insert into the inferior portion of this ridge, so an incision below this anatomic landmark will be impacted by the muscle movement beneath it. A stretched scar in this location is extremely difficult to repair because re-excision, even with undermining and layered closure, tends to heal with a wider scar. In addition, one is more likely to cut through fascia with a low donor incision; and once the fascia has been violated, the risk of widening the scar increases considerably.41 To compound the problem, some patient’s nuchal hair thins dramatically over time, rendering low donor scars visible.
Harvesting hair too high in the donor area risks lack of permanence of the transplanted hair because this hair may be subject to androgenic alopecia. The donor scar may also become visible if the donor fringe narrows further. In addition, a high donor scar has a greater risk of widening compared to one placed at the level of the occipital protuberance. Incisions made too high are best left untreated, unless poor surgical technique has been identified as the cause. The temptation to transplant permanent donor hair into a high scar should be approached with caution, as progressive balding may isolate the hair-bearing scar, presenting new cosmetic problems.
In the case of young patients with traumatic scars and hair-loss patterns that are still unclear, FUE can function as a hedge against this risk. If the hair is harvested from the immediate vicinity of the scar, any future balding will affect the transplanted hair in the scar at the same rate as the hair surrounding it.
Using a multi-bladed knife
In order to save time, doctors performing large transplants may use a multi-bladed knife (a handle with three or more blades attached) for harvesting donor tissue. The resulting pre-sliced multiple thin strips are much easier to work with than a single, intact strip. Unfortunately, harvesting this way causes unacceptable levels of follicular transection and destroys naturally occurring follicular units. This technique is incompatible with FUT.27
Over-aggressive harvesting in FUE
In general, FUE does not yield as many grafts as FUT, so the attempt to harvest large number of grafts with FUE equivalent to what one is used to with FUT, can put patients at risk of visible scarring. Over-aggressive harvesting in FUE comes in two forms; harvesting from an area that is too broad and removing too much hair in a specific area. When the extraction is performed either too high or too low on the scalp, the small pin-point scars may become visible over time and the hair that had been transplanted from these areas will disappear. When too many follicular units are removed per unit area, either in one session, or over repeat procedures, the donor area can take on a moth-eaten appearance. This can severely limit the ability to wear one’s hair short and defeat the purpose of performing FUE.
Crushing grafts during insertion
Proper placing technique necessitates the use of forceps to grasp the graft by the fat below the bulb or by the dermis alongside the hair shaft in order to avoid damaging the germinative components of the follicle. Though placers often exercise care while initially grasping the graft, there is a tendency to become rougher when repositioning the forceps for further inserting, replacing a popped graft, or transferring grafts from the holding solution to the fingers. As follicular units and other small grafts are particularly susceptible to crush injury, improper handling can more than negate the benefits of careful stereomicroscopic dissection.94,95
Allowing grafts to dry
A study using electron microscopy has shown that desiccation is by far the most significant form of injury to grafts and that this makes them much more susceptible to other forms of injury, such as mechanical trauma and warming. Grafts should therefore be kept well hydrated in the designated holding solution from the moment the tissue is harvested until the time they are inserted into the scalp.93
Placing the frontal hairline too far forward (too low)
Despite the fact that individual follicular units at the hairline in themselves look natural, their proper placement is no less important than in traditional grafting. The frontal hairline should be placed no lower than 1.5 cm above the upper brow crease.1,7 If the underlying skin is normal, follicular units placed too low can be removed with an alexandrite (755 nm) or diode (800 nm, 810 nm) laser. Electrolysis is more difficult and time-consuming with transplanted follicles, but should also be considered. Punch excision is too imprecise for very small grafts, and correction using FUE with wounds left to heal by secondary intention, risks visible scarring.
Creating a hairline that is too broad
Since significant temporal recession is characteristic of a normal adult man’s hairline, a broad, flat transplanted hairline will not age well and can cause cosmetic problems if baldness becomes extensive. The treatment is the same as with low hairlines, but if grafts larger than follicular units were used, and/or if there is scarring of the recipient skin, punch excision with reutilization of the hair should be considered.
Angling hair in the wrong direction
As noted earlier, in the front and top part of the scalp, hair grows in a distinctly forward direction, changing to a radial pattern as it approaches the crown. Hair emerges from the scalp at an acute angle, with the hair lying practically flush to the skin at the temples.
There has been a tendency among some hair restoration surgeons to transplant grafts perpendicular to the skin – probably due to the fact that the mechanics of the old plug procedures made sharp angling technically difficult. The cosmetic consequence of this is most apparent at the frontal hairline. When the hair is perpendicular, the viewer’s eye is guided to the base of the hair shaft where it inserts into the skin; conversely, when hair is transplanted in its natural, forward-pointing position, it is bowed by grooming and the eye settles on the body of the hair shaft.
When grafts at the frontal hairline are transplanted in a radial direction, combing the hair in any style becomes problematic as the transplanted hair fans outward and becomes unmanageable. When the hair is pointed forward, it can be groomed as a single unit increasing the illusion of fullness. As with low or broad hairlines, hair that is angled in the wrong direction, particularly in the frontal hairline, should often be removed rather than camouflaged.
Attempting to cover an area that is too large
Attempting to cover an area greater than the donor supply can adequately fill may leave cosmetically important areas thin or un-transplanted. As a general guide, the first region of the scalp to bald is generally the area where you should be most hesitant to transplant. Recession at the temples and thinning in the crown are usually the earliest manifestations of baldness, but they are acceptable, especially as a person ages, so these areas may be left untransplanted. The central forelock region, however, is generally late to bald, but when balding occurs the patient loses the frame to his face and its restoration becomes essential.6
Whether or not these areas need coverage at the time of the initial transplant, an adequate amount of hair must always be reserved for critical areas, such as the forelock and top of the scalp. If donor reserves are limited, the transplantation of less critical areas should be postponed or avoided all together.6
SUMMARY
Developed over the past two decades, FUHT has emerged as the gold standard in surgical hair restoration. In conserving donor hair, achieving optimal coverage, and creating a natural look, FUT and FUE represent a significant advance over earlier methods of hair restoration. Appropriately, the techniques also demand considerably more from its practitioners. Surgical teams must develop the skill and stamina for the delicate handling of large numbers of follicular unit grafts, while surgeons must cultivate a keen aesthetic sensibility with regard to transplant design and graft placement.
In view of the important psychological aspects of hair loss, follicular unit hair transplantation requires a thorough preoperative assessment to understand the patient’s expectations, a careful examination to determine if surgery is appropriate, and, most importantly, the establishment of realistic goals. If the surgical route is chosen, meticulous attention to detail is required in every aspect of the procedure in order that these goals may be realized. It is a daunting task for the hair restoration surgeon and team to develop the necessary expertise for perfecting FUHT; but when they do, the procedure can benefit patients for their lifetime.